3-Azido-L-alanine HCl
Azido-modified amino acids for bioconjugation with alkyne reporters
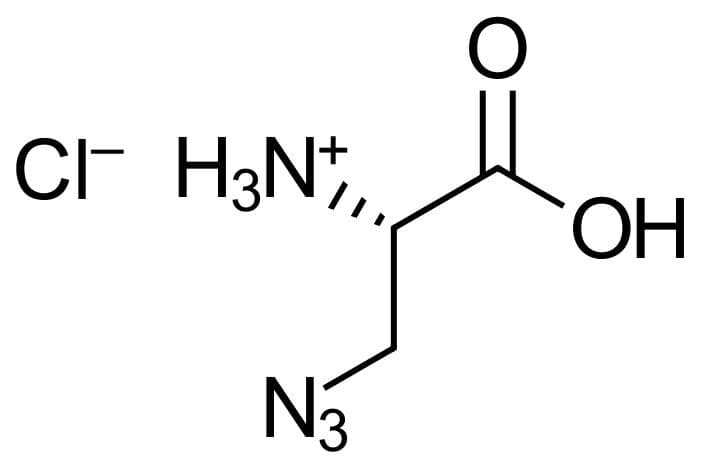
-
3-Azido-L-alanine HCl as a azide-derivatized amino acid is highly interesting e.g. in the area of peptide synthesis as a powerful tool in the development of new therapeutics and biochemistry research. Such modified amino acids can easily undergo click reaction.
LITERATURE
Mapping the Ligand-Binding Site on a G Protein-Coupled Receptor (GPCR) Using Genetically Encoded Photocrosslinkers, Grunbeck et al., 2011, Biochemistry, Vol. 50, p. 3411-3413.
https://doi.org/10.1021/bi200214r
Potent In Vitro Peptide Antagonists of the Thrombopoietin Receptor as Potential Myelofibrosis Drugs, M. Szabo et al., 2021, Advanced Therapeutics, Vol. 4(3), 2000241.
https://doi.org/10.1002/adtp.202000241
Redirecting RiPP Biosynthetic Enzymes to Proteins and Backbone-Modified Substrates, J. A. Walker et al., 2022, ACS Cent. Sci., Vol. 8(4), p. 473–482.
https://doi.org/10.1021/acscentsci.1c01577
Mutagenicity of 3-azido-1,2-propanediol and 9-(3-azido-2-hydroxypropyl)-adenine in repair deficient strains of Escherichia coli, P. Grúz et al., 1993, Mutation Research Letters, Vol. 303(1), p. 1-9.
FAQ
-
Is it possible to generate azide- or alkyne-modified peptides?
With modified amino acids azide- or alkyne-modified peptides can be prepared by solid-phase synthesis.
-
How can de novo protein biosynthesis be monitored?
De novo protein biosynthesis can be monitored by feeding of metabolite analogues (so-called metabolic labeling) and subsequent click reaction. Azido-homoalanine for example is recognized as a methionine analogue and is incorporated into de novo synthesized proteins in methionine-free medium conditions. The resulting proteins contain azide moieties and thus can be detected after click to an alkyne-containing reporter molecule (e.g. a fluorescent dye). This non-radioactive method has major practical advantages compared to traditional 35S amino acid incorporation methods.
Alternatively, O-propargyl-puromycin is efficiently incorporated into proteins during de novo protein biosynthesis and can be used in complete medium. The resulting alkyne protein fragments can be detected via click to azide-containing reporter molecules. -
What click conditions should be used for protein click reactions?
A catalyst system based on CuSO4 and sodium ascorbate is recommended in combination with dye azides to label alkyne-modified proteins. Please also refer to our general Click protocols for more details.
Due to the 20 (21) amino acids that are the building blocks of proteins, the physicochemical properties of proteins are more diverse compared to oligonucleotides, which are just composed of 5 major building blocks. Therefore, finding the optimal click conditions is more difficult compared to oligonucleotides and labeling rates are usually lower. Please note that despite these difficulties detection applications (e.g. de novo protein biosynthesis detection) are easily feasible.
-
Is it possible to generate azide- or alkyne-modified peptides?
-
-
Molecular Formula
C3H6N4O2 *HCI
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
2-8 °C, dry, hygroscopic!
-
Molecular Weight
130.11 g/mol * 36.45 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
white to off-white crystalline powder
-
CAS Number
1620171-64-3 (hydrochloride salt)
105661-40-3 (free acid) -
Additional name
H-L-Aza-OH *HCl; H-L-Dap(N3)-OH*HCl; H-L-Ala(N3)-OH*HCl; 3-Azido-L-alanine hydrochlorid; (S)-2-Amino-3-azidopropanoic acid hydrochlorid
-
Molecular Formula

