DNA crosslinking: Mechanism, applications & significance in molecular research
DNA crosslinking occurs when specific agents (DNA crosslinking agents) bind to nucleotide residues of DNA to form a covalent bond between them. This DNA crosslinking can occur within the same strand or between the two strands of the double helix. DNA crosslinking agents cause covalent crosslinking of DNA strands, abnormal base pairing or DNA strand breaks, thereby preventing the cell from dividing. The use of DNA crosslinking agents was born out of the horror of the use of chemical weapons in the Second World War. However, decades later, DNA crosslinking agents are used instead as chemotherapeutic agents in the treatment of leukaemia and solid tumours.
The mechanism of DNA crosslinking
The first anti-cancer drugs and the most popular DNA crosslinking agents are alkylating agents, which are still used in cancer treatment today. Alkylating agents attach an alkyl group (CnH2n+1) to DNA bases (guanine, adenine, cytosine, thymidine), thereby changing the configuration of the DNA strand. All nitrogen and oxygen atoms of these nucleotide bases are nucleophilic, with the exception of the nitrogen atoms involved in nucleoside binding (N9 or N1 in purines or pyrimidines). DNA crosslinking alkylating agents are electrophilic and bind covalently to electron-rich functional groups. Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene. DNA base alkylation sites are:
Positions of guanine N-7, N-1 and O-6
Positions of adenine N-1, N-3, and N-7
Position of cytosine N-3
Position of thymidine O-4
DNA crosslinking agents make the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. Based on the number of alkyl groups in their molecule, DNA crosslinking alkylating agents are classified as monofunctional, bifunctional and polyfunctional. There are several groups of alkylating agents such as nitrogen mustard analogs, alkylsulfonates, hydrazines and triazines nitrosureas and metal salts.
Types of DNA crosslinking
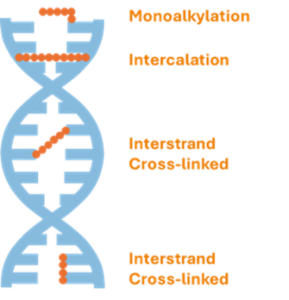
Interstrand & intrastrand crosslinking
As shown in the figure below, DNA crosslinking alkylating agents can cause monoalkylation or intercalation between two DNA strands. Bifunctional alkylating agents can form bridges within a single DNA strand (intrastrand crosslinks) or between two complementary strands of the double helix (interstrand crosslinks (ICLs)), as well as crosslinks between DNA and associated proteins. DNA crosslinking agents prevent the strands from unwinding and separating. As this is necessary for DNA replication, cells cannot divide – perfect for chemotherapeutic agents in cancer treatment.
DNA-protein crosslinking
Crosslinks also occur between DNA and protein. DNA replication is blocked by crosslinks, which causes replication arrest and cell death if the crosslink is not repaired. By combining the cytotoxic power of DNA crosslinking alkylating agents with the unique properties of proteins such as antimicrobial peptides, dual-action therapeutics are available. This may offer a new and more effective approach to fighting cancer.
Applications of DNA crosslinking in research
DNA crosslinking in cancer research
DNA crosslinking agents are substance that binds DNA nucleotides together and block DNA synthesis. Malignant cancer cells, tend to multiply rapidly, invade surrounding tissue and form metastases in other parts of the body. That’s why DNA crosslinking agents are highly interesting in cancer treatment as anticancer agents to stop DNA synthesis. How does it work? The DNA crosslinking agents lead to a covalent crosslink which is can not repaired and stops replication to reach arrest and cell death. Examples of such anticancer agents are nitrogen mustard analogs wich are a type of alkyl halides, platinum compounds, mitomycin C and psoralens. Some of these modified compounds capable of crosslinking DNA are highly important and a tool in the development of cancer therapies.
DNA crosslinking in genomic studies
Gene regulation is partly controlled by transcription factors within chromatin. This is of great interest for the analysis and study of gene regulation. Chromatin (in the cell nucleus) is made up of DNA wrapped tightly around histone proteins to form nucleosomes. The chromatin structure regulates access to genes and therefore plays an important role in controlling gene expression. The individual nucleosomes are connected by a short stretch of linker DNA, forming a string of beads that are in turn packaged together by special crosslinking proteins. DNA crosslinking proteases play a role in proteolysis, in the degradation of excess histones during DNA replication or in the control of DNA replication.
Crosslinking in DNA repair mechanisms
Oxidative or radical mediated (e.g., γ-radiolysis, UV photolysis) DNA damage has been implicated in ageing and a wide range of diseases such as cancer and Alzheimer. Researches have been made in understanding the chemistry of DNA damage by e.g. independently generating radical intermediates in the biopolymer. In cells, nuclear DNA is typically bound by histone proteins, such as in chromatin, and transiently by proteins that regulate biochemical processes. How and whether protein binding affects the reactivity of DNA to radicals is unknown and not well understood. Some modified nucleosides capable DNA crosslinking are used to study DNA damage and repair. The disruption in base stacking could significantly alter the reactivity of nucleotide radicals formed at specific DNA positions. DNA crosslinking agents show how protein binding significantly alters the reactivity of DNA radicals.
Common DNA crosslinking agents
In the last decades great steps forward have been made in the development of inducible DNA crosslinking reagents. Novel methods but also new precursors have been discovered to induce DNA interstrand crosslinking formation, including photoinduction, H2O2, fluoride, NaIO4, singlet oxygen (1O2), enzyme induction and as well as click chemistry. H2O2-activated or enzyme induced DNA crosslinkers have been used to target cancer cells. DNA crosslinking induced by photo irradiation or click chemistry has been used to construct nanomaterials. Several photoswitchable DNA crosslinking reagents are available for DNA manipulation, sequence specific RNA editing and selection, and single nucleotide polymorphism based genotyping. The techniques developed may either improve DNA crosslinking efficiency and selectivity or extend the application in medical area. DNA crosslinking agents such as alkyl halides (nitrogen mustard and its analogs), quinone methides and carbocations are the major electrophiles are powerful DNA cross-linking agents for treatment of various cancer diseases.
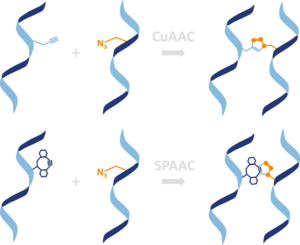
Interstrand DNA crosslinking agents based on click chemistry
Click reactions like the Cu[I] catalyzed alkyne azide cycloaddition (CuAAC) or the strain promoted alkyne azide cycloaddition (SPAAC) are bioorthogonal and highly tolerant of a variety of functional groups. These conjugation reaction is fast, and high yielding, very efficient at ambient temperature, tolerant of other functional groups and require minimal work-up and purification and can be performed in a variety of solvents, including water. Thus, click chemistry is ideal for covalently DNA crosslinking and led to high cross-linking efficiency.
There are several modified nucleic acids and various linker for DNA crosslinking available containing alkyne and azido groups for linking complementary DNA strands. If you want to test alkyne moiety containing nucleosides that can be incorporated into DNA and used for DNA crosslinking via copper-free or copper catalysed reaction then check out our products.
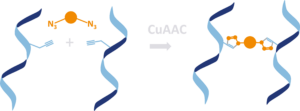
To cross-link two DNA strands containing each e.g. alkyne-modified nucleosides a “bis-click” reaction has been developed wereby a bis-azides or bis-DBCO linker were used for “bis-click” reaction. The DNA crosslinking reaction is highly efficient and proceeds almost quantitatively without side product formation.
The use of customizable oligonucleotides could be used to develop DNA crosslinking strategies for single nucleotide polymorphism analysis without the need for expensive equipment and reagents. Oligonucleotides with ethynyl and octadiynyl groups attached to uracil bases can be synthesised and cross-linked to complementary azide-labelled oligonucleotide strands to form stable DNA duplexes.
DNA crosslinking techniques in the laboratory
Photoinduced DNA interstrand crosslinking is a method that offers the possibility of orthogonality, which is attractive for biological applications. Three common mechanisms are involved in photoinduced DNA crosslinking, including [2+2] photocycloaddition using modified nucleosides such as p-stilbazole analogue, coumarin modified thymidine or 3-cyanovinylcarbazole. Another technique for DNA crosslinking is alkylation via the formation of quinone methides, which are highly active electrophiles. The third mechanism for photoinduced DNA crosslinking is via radical and cation trapping for carbocation formation. Apart from photoinduced DNA interstrand crosslinking, various chemical agents are used for DNA interstrand crosslinking, via H2O2 in the presence of arylboronate or boronic acid, via fluoride in the presence of silyl protected phenol group, via NaIO4 in the presence of phenyl selenide or via singlet oxygen to generate reactive enal species. In addition to these techniques, DNA crosslinking can be activated by enzymes. DNA crosslinking can be induced by reductases such as nitroreductases. Finally, DNA interstrand crosslinking is based on click chemistry.
Storage & stability of DNA crosslinking agents
Most DNA crosslinking agents are best stored at refrigerator temperatures (4°C) away from UV or other light. Some may require freezer storage (-20°C) for longer shelf life. However, different agents have different stability and may require specific storage conditions, please check their SDS/MSDS.