Fluorescent dyes & their spectra: A comprehensive overview
Fluorescent dyes are molecules that absorb light at one wavelength and emit it at a longer one making them glow under UV or blue light.
Fluorescent dyes have revolutionized life sciences due to their versatility and sensitivity. Key advantages include:
- Sensitive & Specific: Detect tiny amounts of biomolecules and label them precisely.
- Live Imaging: Visualize cells and tissues without damage.
- Multiplexing: Use multiple dyes to track different targets simultaneously.
Introduction to fluorescent dyes
The development and growing role of fluorescent dyes in molecular research have transformed how scientists study biological systems at the molecular and cellular levels.
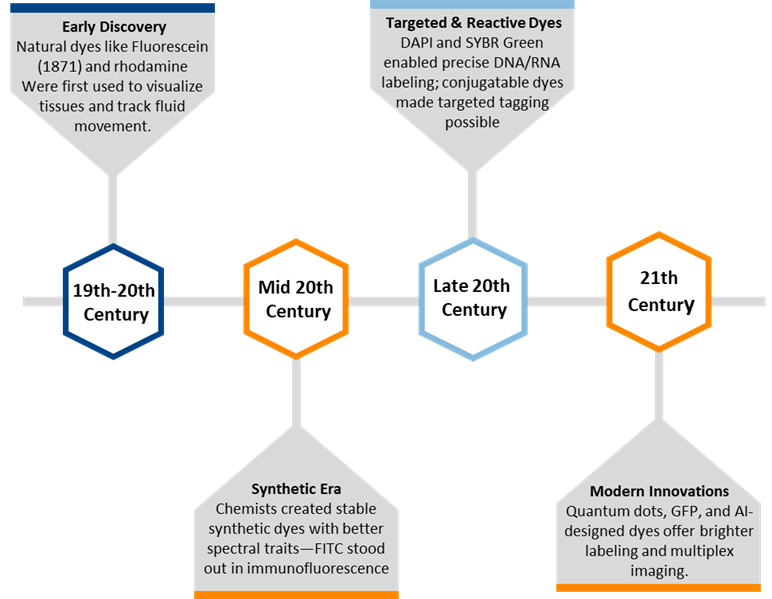
Figure 1. Development history of fluorescent dyes
Fluorescent dyes work by absorbing light of shorter wavelengths, typically in the UV or blue spectrum, which excites their electrons to a higher energy state; as these electrons return to their ground state, the dye emits light of a longer wavelength, producing fluorescence. This process, known as the absorption–emission cycle, is visualized through the dye’s absorption spectrum (showing which wavelengths it absorbs) and emission spectrum (showing what it emits), with the difference between these wavelengths called the Stokes shift, a key feature that enables clear signal detection in applications like microscopy, diagnostics, and molecular imaging.
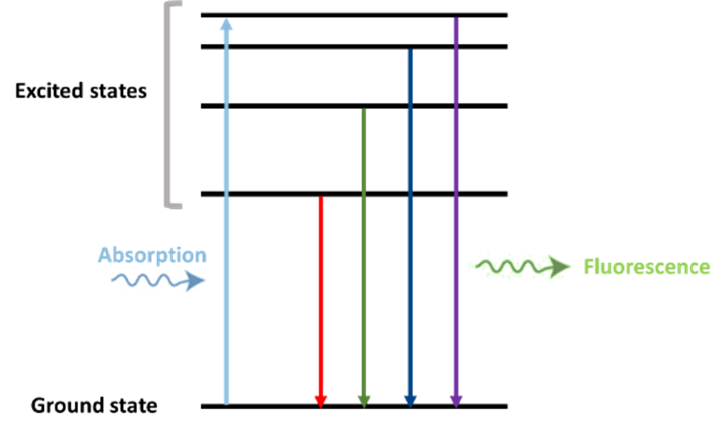
Figure 2. Absorption and emission pathways
What are fluorescent dyes?
The chemical structure & mechanism of fluorescent dyes
Fluorescent dyes absorb light, exciting their electrons to a higher energy state; as the electrons return to their original state, they emit light at a longer wavelength—a process called fluorescence. This energy shift, known as the Stokes shift, depends on the dye’s structure and environment, making these dyes ideal for labeling and tracking biological molecules in imaging techniques like fluorescence microscopy
Conjugated systems, chains of alternating single and double bonds, are key to fluorescent dyes because they let electrons absorb and emit light efficiently. This electron delocalization ensures bright fluorescence, stability during repeated use, and tunable color based on molecular structure. Without conjugation, dyes wouldn’t glow or last long in imaging applications.
The main types of fluorescent dyes
The following table highlights widely used organic dyes that play key roles in fluorescence imaging, cellular staining, and various analytical chemistry techniques.
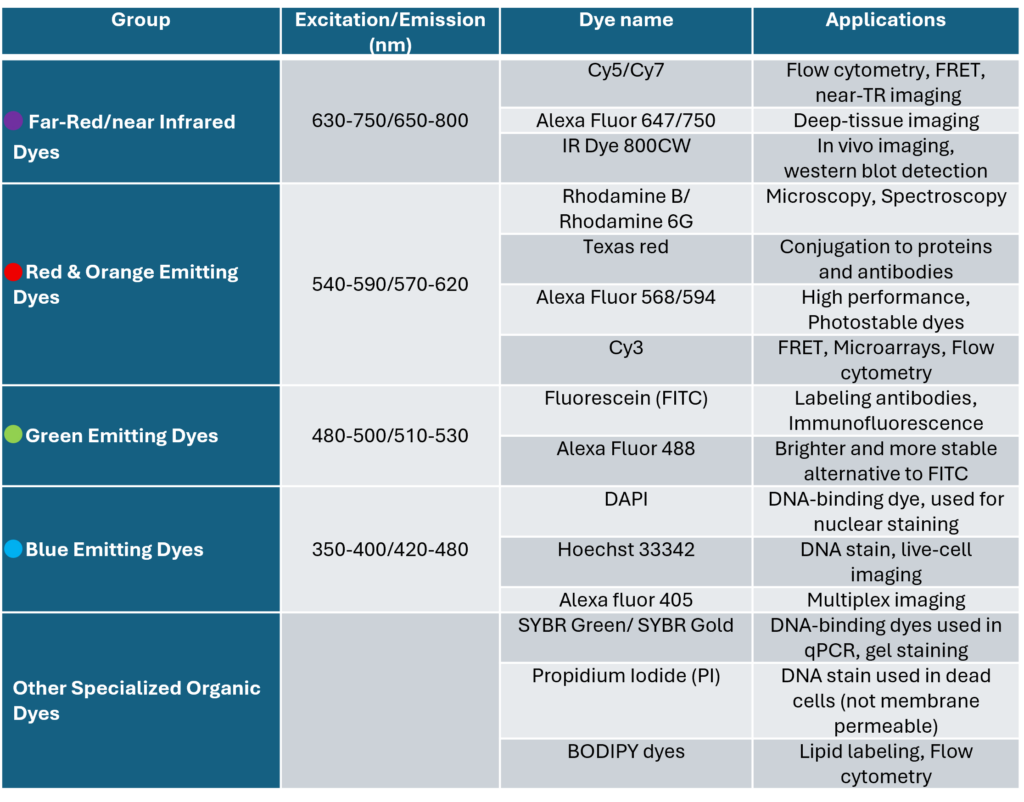
Learn more about Flow Cytometry Glossary here: Flow cytometry Glossary
Synthetic dyes offer high precision, strong brightness, and flexible labeling, making them ideal for fixed samples and short-term in vitro assays. In contrast, fluorescent proteins are genetically encodable and ideal in long-term, real-time live-cell imaging, allowing dynamic tracking of proteins within living systems.
The selection of a fluorescent dye depends heavily on application-specific needs, particularly its spectral properties like excitation and emission wavelengths.
- Multiplex imaging: dyes with distinct, non-overlapping emission spectra help separate multiple targets clearly.
- Quantitative techniques: Such as qPCR, FRET, or flow cytometry, dyes must offer high quantum yield, narrow emission peaks, and strong signal stability for accurate measurement.
- Live-cell imaging: Dyes with low phototoxicity and high photostability, often emitting at longer wavelengths, are ideal to reduce cell stress and allow prolonged observation.
- Matching dyes to instrumentation: is also crucial. Selected dyes must align with available lasers or filters for optimal excitation and detection.
- Effective targeting: requires dyes to bind specifically, DNA dyes like DAPI or SYBR Green, protein tags such as FITC or Alexa Fluor antibodies, and membrane stains like DiI or DiO.
- Applying as biosensor: In some applications, environment-sensitive dyes respond to changes in pH, ions, or polarity, making them useful as biosensors.
Ultimately, thoughtful dye selection ensures clear signals, minimal background interference, and reliable performance tailored to the experimental setup.
Understanding fluorescent dye spectra
Absorption & emission spectra
Fluorescent dyes selection depends on its excitation and emission maxima wavelengths where the dye absorbs and emits light most effectively. The gap between them, called the Stokes shift, enables clear signal detection. These maxima guide essential aspects of experimental design:
- Optimal Signal Intensity: Using excitation light near λₑₓ ensures strong fluorescence.
- Accurate Detection: Emission filters should match λₑₘ to capture a sharp signal.
- Multiplex Imaging: Dyes with non-overlapping spectra prevent cross-talk in multi-label experiments.
- Instrument Compatibility: Selected dyes must match the lasers and filters of microscopes, cytometers, or readers.
- Sensitivity and Quantification: High quantum yield and narrow emission peaks enhance precision in assays like qPCR, FRET, or ELISA.
Fluorescent dyes spectra play a central role in configuring fluorescence-based instruments. Each dye has unique excitation and emission maxima, and instruments must match these to function effectively. Excitation filters and light sources (like lasers or LEDs) are selected to deliver light at or near the dye’s excitation peak. Once fluorescence is emitted, emission filters capture light around the dye’s emission maximum, while dichroic mirrors separate incoming excitation light from emitted signals based on the Stokes shift. In setups using multiple dyes, choosing filter sets tailored to each dye’s spectral signature ensures clean signal separation and minimizes overlap, essential for accurate multiplex imaging or detection. By aligning all optical components with the dye’s spectral behavior, researchers get the strongest signals with the least background interference.
Challenges & strategies in multiplexing
Signal bleed-through, also called spectral overlap or cross-talk, occurs when light from one source is mistakenly detected in a channel intended for another. This overlapping can lead to inaccurate measurements and compromise the integrity of multi-spectral data. It can happen through:
- Overlapping spectra: When two fluorophores have emission or excitation wavelengths that are too similar, their signals can interfere with each other.
- Imperfect detection systems: Filters or detectors may not completely separate these overlapping signals.
- Unintended detection: As a result, light from one fluorophore can spill into the detection channel of another.
Spectral separation is the strategic selection of fluorescent dyes whose:
- Excitation and emission spectra are well separated (ideally ≥30–50 nm apart)
- Emission profiles minimize overlap across detection channels
This enables simultaneous detection of multiple targets such as proteins, nucleic acids, or cell types without compromising accuracy.
Spectral separation is crucial since it can lead to:
- Accurate identification: Ensures each dye labels one target and is detected in a dedicated channel
- Minimizes Crosstalk: Reduces bleed-through between channels, preventing signal confusion
- Enhances Quantification: Allows precise intensity measurements for ratiometric and multi-analyte analysis
- Enables Reliable Multiplexing: Supports complex experiments like immunofluorescence, FRET, or flow cytometry
Applications of fluorescent dyes in scientific research
Molecular labeling techniques using fluorescent dyes
Fluorescent dyes are used to tag biological molecules and structures:
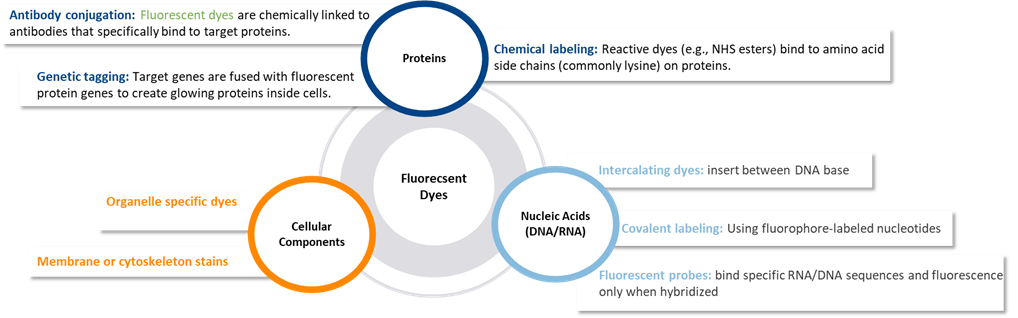
Fluorescent dyes are powerful tools in lifescience, enabling researchers to see, follow and measure biological molecules and processes in real time or fixed samples.
- Imaging: Fluorescent dyes are used to illuminate specific biological targets such as DNA, proteins, or organelles allowing scientists to visualize cellular structures under a fluorescence microscope. This results in sharp, color-coded images that reveal the architecture, location, and interactions of components in 2D or 3D.
Such imaging enables:
- Colocalization studies
- Cell type identification
- Tissue mapping
- Tracking: Fluorescent dyes are powerful tools for monitoring dynamic biological processes in real time, particularly in live-cell imaging.
They enable scientists to:
- Visualize movement and changes of cells, organelles, or molecules over time
- Track biological events like cell division, migration, and intracellular transport
- Study molecular dynamics, including signaling pathways and immune responses
- Molecular Quantification: Fluorescent dyes enable precise measurement of biomolecule concentration or activity by producing light intensity proportional to the target’s amount.
This principle is foundational in techniques like:
- qPCR, for quantifying gene expression using fluorogenic probes (e.g., SYBR Green)
- Protein and enzyme assays, for measuring activity or concentration
- Multiplex detection, allowing simultaneous quantification of multiple analytes
Quantitative data is obtained by plotting or calculating fluorescence intensity, delivering high-sensitivity results critical for diagnostics, research, and molecular biology.
Diagnostic & research applications
Fluorescent labeling is a cornerstone of modern molecular and cellular biology, offering high sensitivity, specificity, and real-time visualization across diverse applications:
- qPCR: in qPCR, Fluorophores like SYBR Green, TaqMan probes, and molecular beacons emit signals during DNA amplification, enabling quantitative gene expression analysis.
- ELISA: in fluorescent ELISA, antibodies are tagged with dyes such as FITC or Alexa Fluor, providing enhanced sensitivity and enabling multiplex detection of proteins and hormones. Immunofluorescence incorporates fluorophore-conjugated antibodies to reveal protein localization in tissues, using either direct or indirect labeling with dyes like Cy3 or TRITC.
- In situ hybridization: especially FISH, employs labeled nucleic acid probes to detect specific DNA/RNA sequences in cells, uncovering gene expression, chromosomal anomalies, or viral genetic material, all while preserving tissue architecture.
Learn more about In situ Hybridization Glossary here: In situ Hybridization Glossary
These techniques share common goals: detecting biomolecules with precision, improving signal clarity, and enabling quantitative insights that are critical for diagnostics, research, and therapeutic development.
Fluorescent dyes are foundational to modern diagnostics. Each dye features defined excitation and emission wavelengths, with factors such as quantum yield, Stokes shift, photostability, and spectral separation playing critical roles in performance.
- High sensitivity depends on bright fluorescence, minimal background (especially avoiding overlap with biological autofluorescence), and narrow emission bandwidths that enhance detection of low-abundance targets.
- High specificity stems from choosing dyes with distinct, non-overlapping emission spectra, enabling accurate multiplexing and reducing cross-talk crucial in assays like multiplex FISH and immunofluorescence.
Best practices include selecting photostable, high-yield dyes, minimizing spectral overlap, and validating dye/filter combinations experimentally. When tailored appropriately, fluorescent labeling dramatically enhances clarity, precision, and reliability in both research and diagnostic platforms.
Selecting the right fluorescent dye
Key selection criteria
Selecting the right fluorescent dyes is essential for optimizing diagnostic sensitivity, specificity and overall experimental success:
Selecting an appropriate fluorescent dye demands careful consideration of its compatibility with both laboratory instrumentation and the biological and chemical reagents used throughout the experiment.
- Compatibility with lab equipment: For optimal fluorescence detection, the dye’s excitation and emission wavelengths should be aligned with the instrument’s filters and light source, for example, FITC pairs well with a 488 nm laser. Detection systems must be sensitive to the dye’s emission spectrum, although older equipment may limit the available options. In multiplexing applications, using spectrally distinct dyes and planning for signal compensation are essential to prevent cross-talk.
- Compatibility with reagents and sample types: is essential for fluorescent dyes. Dyes must conjugate effectively to biomolecules, using linkers like NHS esters or maleimides, while remaining stable in buffers across pH and salt conditions. In qPCR, dyes should resist thermal cycling and avoid enzyme interference. Sample type matters too: fixed tissues need photostable dyes (e.g., Alexa Fluors), live cells require biocompatible, cell-permeable dyes, and flow cytometry demands low-autofluorescence options.
Functionalization options
Fluorescent dyes can be chemically tailored with reactive functional groups to enable precise and stable labeling of biomolecules, an essential feature for diagnostics, imaging, and analytical applications. These modifications allow dyes to covalently attach to specific targets such as proteins, nucleic acids, glycans, or small molecules, enhancing both specificity and durability of the fluorescence signal. Common reactive groups include NHS esters (targeting amines), maleimides (for thiols), isothiocyanates, hydrazides (binding aldehydes), and click chemistry tags like azides or alkynes for site-specific conjugation.
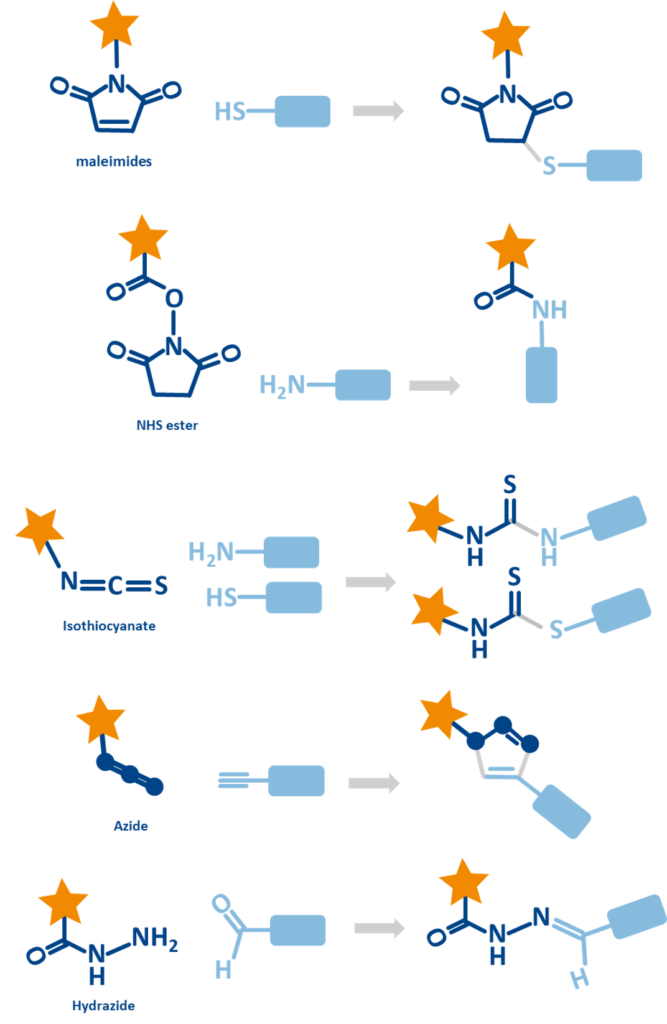
These enable a variety of workflows, from antibody labeling in immunofluorescence and flow cytometry to nucleic acid tagging in qPCR or FISH, and even glycoprotein profiling using oxidized sugars.
Most fluorescent dyes are designed to be highly compatible with standard chemical conjugation methods, making them easy to integrate into experimental workflows. Conjugation strategies rely on these reactive groups to form covalent bonds with target molecules:
- Amine-based conjugation: Dyes with NHS esters or isothiocyanates are widely used for labeling antibodies and proteins through their amine residues.
- Thiol-based conjugation: Maleimide dyes pair well with thiol-containing proteins or peptides, offering site-specific labeling.
- Click chemistry: Azide or alkyne functionalized dyes are ideal for high-specificity labeling through copper-catalyzed or strain-promoted cycloaddition reactions.
Learn more about Click Chemistry Glossary here: Click Chemistry Glossary
- Hydrazone formation: Hydrazide dyes label oxidized glycans or aldehyde-functional targets commonly used in glycoprotein studies.
These dyes are supplied pre-functionalized and compatible with commercial bioconjugation kits, simplifying the process and ensuring consistency across applications.
Storage & handling of fluorescent dyes
Best practices for laboratory use
Proper handling of fluorescent dyes is essential to prevent photobleaching and degradation, ensuring stable fluorescence and reliable experimental results
- Protect from light: Storing dyes in amber or foil-wrapped containers and handle under dim or red light to prevent photobleaching.
- Maintain low temperatures: Storing dyes at –20°C or –80°C and aliquot to avoid repeated freeze–thaw cycles.
- Work efficiently: Minimizing the time dyes spend at room temperature and exposed to light during experiments.
- Add stabilizers if compatible: Antioxidants such as Trolox or ascorbic acid can help reduce oxidative degradation.
- Avoid harsh conditions: Protecting dyes from oxidizers, extreme pH shifts, and strong detergents unless verified for stability.
The following points outline the recommended solvents and usage conditions to ensure optimal performance of fluorescent dyes:
- Choose appropriate solvents: Dissolving dyes in anhydrous DMSO, DMF, or ethanol when recommended. Aqueous-soluble dyes may be used in water-based buffers like PBS or Tris.
- Control pH: Using buffers that maintain pH in the dye’s optimal range (typically pH 7.2–7.4), especially for maintaining reactive group stability.
- Check solvent compatibility: Ensuring chosen solvents don’t interfere with labeling, target molecules, or detection systems.
- Filter solutions: Using filters (0.2 µm) to remove particulates that may scatter light or interfere with detection.
Applying these best practices enhances fluorescence signal integrity, reduces background noise, and supports reproducible results, especially critical for imaging, qPCR, flow cytometry, and multiplex assays.
Storage requirements
To preserve fluorescent dyes integrity and prevent photobleaching or degradation:
Temperature Management
- Short-term storage (using within a week): Storing aqueous dye solutions at 4°C.
- Long-term storage: keeping dyes at –20°C (standard dyes in organic solvents) or –80°C (sensitive dyes or extended storage)
- Aliquot dyes into small volumes to prevent repeated freeze–thaw cycles.
Light Protection
- Fluorescent dyes are highly light-sensitive, and exposure can lead to rapid photobleaching. To prevent this, they should be stored in amber vials, foil-wrapped tubes, or opaque containers placed inside light-blocking boxes.
- Handling under dim or red/green safe lighting, and minimizing light exposure during sample preparation and labeling.
Taking these precautions helps maintain dye brightness, extend shelf life, and ensure dependable results in imaging, diagnostics, and analytical workflows.
baseclick offers a wide range of fluorescent dyes for labeling biomolecules using mild and efficient click chemistry.


