
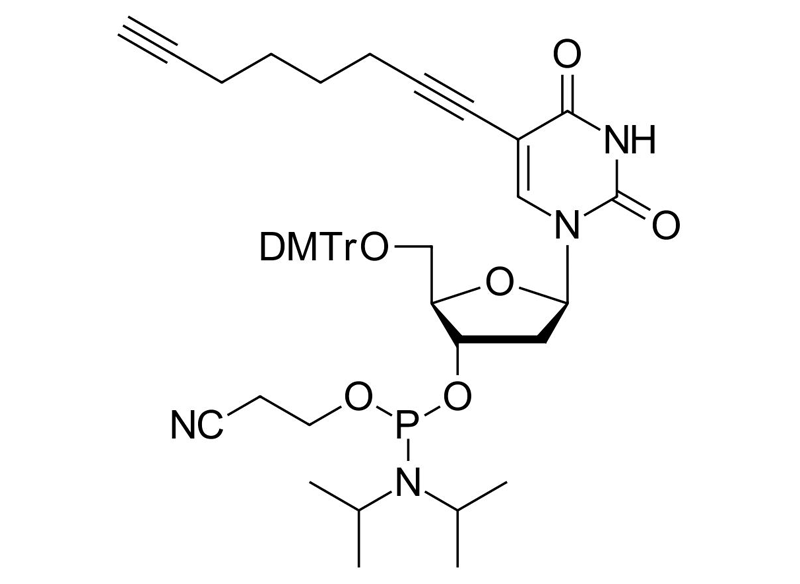
C8-Alkyne-dU-CE Phosphoramidite
Clickable phosphoramidite for Oligo synthesis
| Size | Catalog No. | Price |
|---|---|---|
| 250 mg | BCA-03-250 | € 240,00 |
| 1 g | BCA-03-1g | € 680,00 |
| 5 g | BCA-03-5g | € 2.500,00 |
Chemical Properties
-
Molecular Formula
C47H55N4O8P
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C dry, inert gas
-
Molecular Weight
834.94 g/mol
-
Purity
≥ 98% (HPLC & 31P-NMR)
-
Physical State
white to off-white / light yellow solid
-
CAS Number
938186-76-6
-
Additional name
C8-Alkyne-dU-phosphoramidite, 5-(Octa-1,7-diynyl)-3`-O-[(2-cyanoethoxy)(diisopropylamino)-phosphono)]-5`-O- (4,4`-dimethoxytrityl)-2`-deoxyuridine
-
Preparation/Handling
C8-Alkyne-dU-CE Phosphoramidite is used under standard conditions for solid phase synthesis of oligonucleotides.
Product Information
Site-specific alkyne-modified nucleoside for oligonucleotide labeling via click chemistry
C8-Alkyne-dU-CE Phosphoramidite is a chemically modified nucleoside designed for efficient, site-specific incorporation into DNA, RNA, or PNA strands during solid-phase oligonucleotide synthesis. Featuring a C8 alkyne spacer at the C5 position of deoxyuridine, this phosphoramidite enables robust bioorthogonal labeling via copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC).
The long, flexible octadiynyl chain significantly improves accessibility for post-synthetic modifications, making this building block ideal for developing functionalized probes, bioconjugates, and labeled oligonucleotides.
Chemical features
Backbone: Deoxyuridine (dU) base
C8 Spacer: Octadiynyl chain for improved flexibility and labeling access
5′-Hydroxyl Protection: DMT group for controlled synthesis
Phosphoramidite Group: 2-cyanoethyl (CE) for high coupling efficiency
This structural configuration supports efficient, stable, and high-yield incorporation into oligonucleotides under standard solid-phase synthesis conditions.
C8-Alkyne-dU-CE Phosphoramidite is a modified nucleoside designed for oligonucleotide synthesis, featuring a C8 spacer that enhances flexibility and accessibility for click chemistry reactions.
Earlier alkynyl-modified phosphoramidites had short, rigid linkers that limited conjugation efficiency and often interfered with duplex stability and hybridization performance.
C8-Alkyne-dU-CE offers:
- Improved click accessibility due to a longer flexible linker
- High retention of hybridization capacity — no disruption of DNA duplex formation
- Slight duplex stabilization, making it suitable for TaqMan and other hybridization-dependent applications
Applications
- Site-specific labeling of oligonucleotides with azide-bearing molecules (fluorophores, peptides, biotin, etc.)
- Development of multiplexed imaging probes
- Construction of click-functionalized TaqMan qPCR probes
- Dual or triple labeling for diagnostics and biosensing
- Functionalization for drug delivery or targeted therapeutics
- Real-time PCR, hybridization assays, FISH, and molecular barcoding
Technical Insight
Published research has demonstrated that oligonucleotides incorporating C8-Alkyne-dU maintain normal duplex stability and can even exhibit slight enhancement of melting temperatures, ensuring compatibility with hybridization-based techniques.
LITERATURE
Nucleosides And Oligonucleotides With Diynyl Side Chains: The Huisgen-Sharpless Cycloaddition “Click Reaction” Performed On Dna And Their Constituents, F. Seela et al., 2007, Nucleosides, Nucleotides & Nucleic Acids, Vol. 26, p. 597–601.
https://doi.org/10.1080/15257770701490308
DNA-Photographie: eine ultraempfindliche Methode zur Detektion von DNA mithilfe photographischer Techniken, D. M. Hammond et al., 2007, Angew. Chem., Vol. 119, p. 4262–4265.
https://doi.org/10.1002/ange.200605023
Transfer Printing of DNA by “Click” Chemistry, D. I. Rozkiewicz et al., 2007, ChemBioChem, Vol. 8, p. 1997–2002.
https://doi.org/10.1002/cbic.200700402
DNA Containing Side Chains with Terminal Triple Bonds: Base-Pair Stability and Functionalization of Alkynylated Pyrimidines and 7-Deazapurines, F. Seela et al., 2006, Chemistry & Biodiversity, Vol. 3, p. 509–514.
https://doi.org/10.1002/cbdv.200690054
Click Chemistry as a Reliable Method for the High-Density Postsynthetic Functionalization of Alkyne-Modified DNA, J. Gierlich et al., 2006, Org. Lett., Vol. 8, p. 3639–3642.
https://doi.org/10.1021/ol0610946
A click chemistry approach to developing molecularly targeted DNA scissors, T. Lauria et al., 2020, Chemistry – A European Journal, Vol. 26(70), p. 16782-16792.
https://doi.org/10.1002/chem.202002860
Supersensitive Multifluorophore RNA‐FISH for Early Virus Detection and Flow‐FISH by Using Click Chemistry, N. Raddaoui et al., 2020, ChemBioChem, Vol. 21(15), p. 2214-2218.
https://doi.org/10.1002/cbic.202000081
Selective targeting and degradation of doxorubicin-loaded folate-functionalized DNA nanocages, S. Raniolo et al., 2018, Nanomedicine: Nanotechnology, Biology and Medicine, Vol. 14(4), p. 1181-1190.

