In Vivo EdU Cell Proliferation Assay for High-throughput Screening
ClickTech In Vivo EdU Cell Proliferation Kit for HTS
| Size | Catalog No. | Price |
|---|---|---|
| Dye 488 / 200 Assays / Size S | BCK-EdU488HTS2+IV-S | € 430,00 |
| Dye 488 / 200 Assays / Size M | BCK-EdU488HTS2+IV-M | € 730,00 |
| Dye 488 / 200 Assays / Size L | BCK-EdU488HTS2+IV-L | € 970,00 |
| Dye 555 / 200 Assays / Size S | BCK-EdU555HTS2+IV-S | € 430,00 |
| Dye 555 / 200 Assays / Size M | BCK-EdU555HTS2+IV-M | € 730,00 |
| Dye 555 / 200 Assays / Size L | BCK-EdU555HTS2+IV-L | € 970,00 |
-
Cell Analysis – In Vivo EdU Cell Proliferation Assays
Cell analysis allows scientists to gain unique insights into cell proliferation, viability, and toxicity, enabling the monitoring of cell response and cell health in cultures after treatment with various stimuli.
Detection of DNA replication is a powerful tool for studying proliferation of both cell populations and individual cells. DNA replication rates can be detected through the incorporation of a nucleoside analog such as BrdU or EdU into DNA. The detection of these two thymidine analogs differs significantly and has implications for test results, potential applications, and multiplexing capability. Compared to other cell proliferation methods, the EdU proliferation assay does not involve radioactive isotopes or antibodies to detect newly synthesized DNA.
The ClickTech EdU cell proliferation assay enables estimates of cell proliferation rates by determining the fraction of cells replicating their DNA. The technology is an in vivo DNA labeling with EdU (5-ethynyl-2′-deoxyuridine), which is cell-permeable and can be administered by injection (intraperitoneal, intramuscular, subcutaneous), orally or by direct incubation in certain organisms (e.g. mice), wherein EdU is incorporated into actively dividing cells.
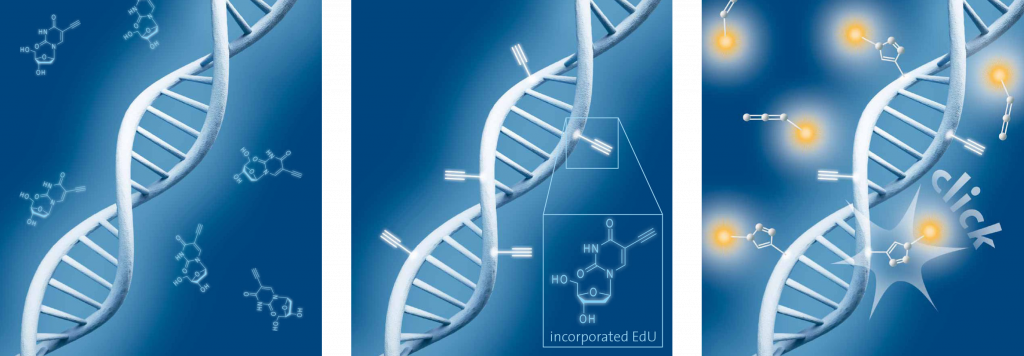
Schematic representation of EdU cell proliferation assays. A) Incubation of cells with EdU. B) EdU is incorporated during active DNA synthesis. C) Detection of cell proliferation via click chemistry, wherein the use of a range of different fluorescent dyes is possible.
Application
This assay enables monitoring genotoxicity, anticancer drug evaluation or cell cycle analysis by direct detection of DNA replication in proliferating cells. The principle of this procedure is similar to that of the BrdU assay, with the difference of the detection reaction. In summary, the thymidine analog, in this case the EdU (5-ethynyl-2′-deoxyuridine), is added to the cell culture media, taken up by the cells and incorporated into newly synthesized DNA during S-phase. This nucleoside contains a reactive alkyne group (instead of the bromine atom in BrdU) and can be conjugated to molecules bearing azide groups through a click reaction. Functional alkyne and azide groups do not occur in natural cell systems and do not react without catalytic reagents. This ensures that the EdU cell proliferation method, based on click chemistry, is highly selective, sensitive and reproducible.
After an incubation period that depends on the specific cell type, the cells are washed, fixed and permeabilized to allow the detection reagents to penetrate. The detection is then carried out via the copper-catalyzed click reaction (also known as Azide-Alkyne Cyclo-addition or CuAAC). Click reactions show high quantitative yields, run under mild conditions and are easy to perform. In order to start the reaction, a click cocktail, containing buffer, catalytic reagents and a fluorescent dye, is added to the cells. Only cells that actively replicate their DNA during the EdU pulse can be detected by this method.
This technology allows an almost unlimited choice of different and easy to synthesize fluorescent dyes and thus offers maximum flexibility in terms of “detection colors” and analytical devices. Cell proliferation can be detected by fluorescence microscopy, flow cytometry and fluorescence plate reader. baseclick currently offers assays for these three analytical methods and up to four standard dyes (488, 555, 594, 647 nm).
Product Overview
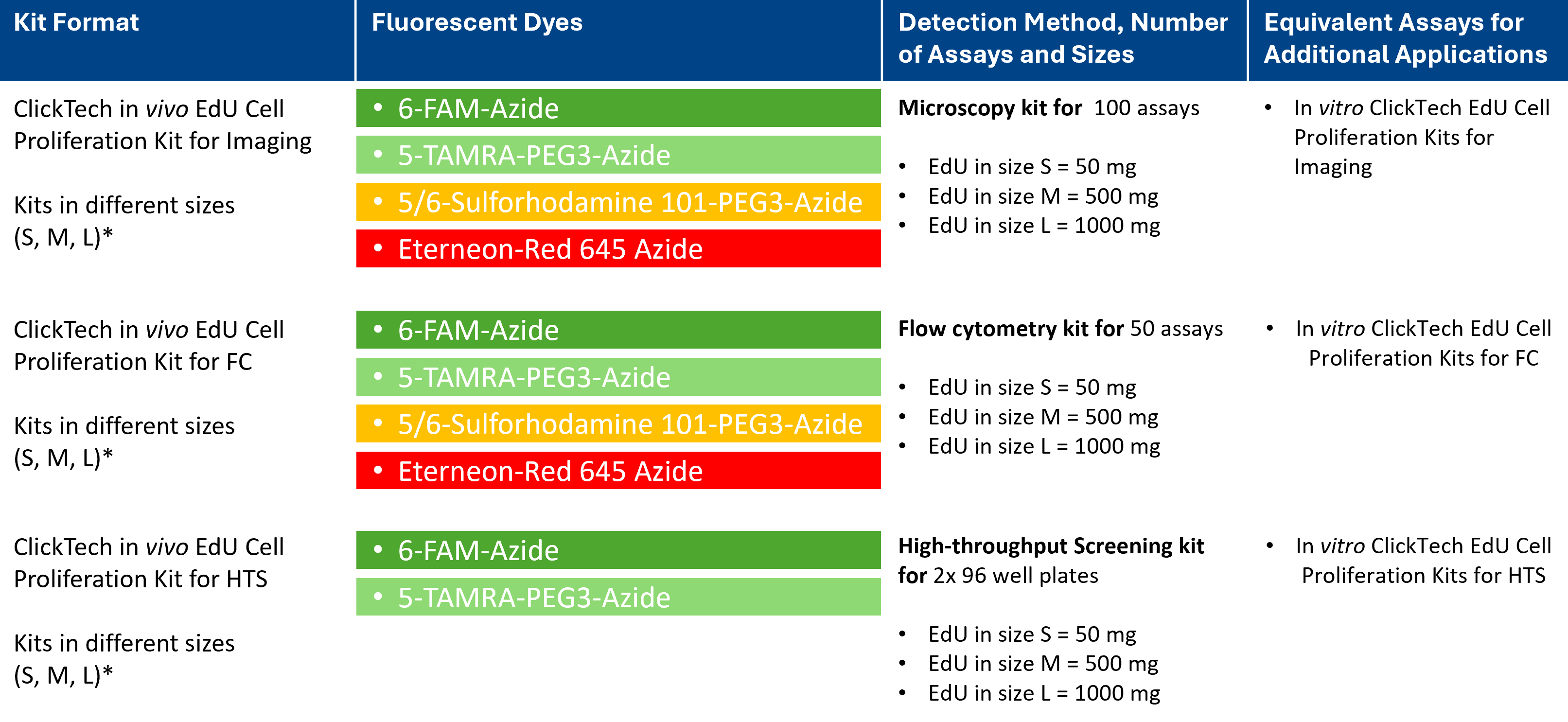
*The three kit sizes S, M and L with increasing EdU content can be selected depending on the animal to be examined or the number of animals.
LITERATURE
An efficient protocol for in vivo labeling of proliferating epithelial cells, C. Michel et al., 2018, J. Immunol. Methods, Vol. 457, p. 82-86.
https://doi.org/10.1016/j.jim.2018.03.015
Cisplatin-induced DNA double-strand breaks promote meiotic chromosome synapsis in PRDM9-controlled mouse hybrid sterility, L. Wang et al., 2018, eLife, Vol. 7, p. e42511.
https://doi.org/10.7554/eLife.42511
FXR Regulates Intestinal Cancer Stem Cell Proliferation, T. Fu et al., 2019, Cell, Vol. 176, p. 1098-1112.
FAQ
-
What type of cells can incorporate EdU?
The EdU cell proliferation assay has been applied to many different cell types and organisms from prokaryotic to eukaryotic. Cell lines such as E. coli, HeLa, HEK, MOLM are arguably among the most routine applications, but also animals, like mouse, rat, the nematode C. elegans, crickets (Gryllus bimaculatus), chicken (Gallus domesticus) and zebra fish (Danio rerio) or even plants (e.g. Arabidopsis thaliana) can be applied.
Cells that possess a pyrimidine pathway that can phosphorylate EdU to the corresponding triphosphate, which is then accepted by the host DNA polymerase for incorporation into DNA during replication. -
Can I perform EdU cell proliferation detection on living cells?
EdU is incorporated into living cells, but the detection reaction must be performed on fixed and permeabilized samples.
-
How does EdU labeling compare to BrdU or the 3H-thymidine incorporation assay?
All three methods enable to determine cell proliferation directly by incorporation of a metabolite analogue and subsequent detection. The 3H-thymidine incorporation assay is very sensitive, but the radioactive compound requires specialized equipment and dedicated lab space for handling. EdU and BrdU assays are non-radioactive alternatives with decreased risk for health and environment. Compared to the BrdU incorporation assay the EdU assay is more sensitive, requires less handling time and needs no harsh DNA denaturing conditions for detection. Therefore, the EdU cell proliferation is also compatible with multiplexing.
-
Can I combine DAPI staining and EdU detection?
Yes, this is feasible. Please note that DAPI staining should be done after the click detection step. Alternatively, SYBR Green DNA staining can be used. But, please note that SYBR green should not be used with dyes of 488 nm wavelengths.
-
When can I safely interrupt the experiment?
It is possible to safely interrupt the protocol after the fixation step. Thereto, remove the fixation solution and wash as suggested by the user manual, then the cells can be stored in buffer at 4° C. Alternatively, the experiment can also be safely interrupted after permeabilization, again as described above.
Please note: It is important to proceed with the experiment if the click cocktail for the detection of the EdU has been prepared already. -
Is antibody staining compatible with the EdU?
Antibody staining is compatible with EdU cell proliferation detection when antibody detection is done after the click detection step. Please be aware of the dye used for EdU detection. Check also the user manual for more information.
-
How to determine the EdU incubation time?
The EdU incubation time depends on the cell type or organism, the applied EdU concentration and the experimental design. For a start it is advisable to refer to a literature protocol (which is close to your experimental setup) and to test the conditions with a low number of samples. As a general guideline we recommend to use a maximum of 10 µM final EdU in the cell culture medium for incubations. For longer incubation (> 1 day) the concentration should be decreased to 1-5 µM.
-
What type of cells can incorporate EdU?
-
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
2-8 °C
-
Physical State
kit system made of different components
-
CAS Number
n.a.
-
Excitation (max)
Dye 488: 496 nm | Dye 555: 546 nm
-
Emission (max)
Dye 488: 516 nm | Dye 555: 579 nm
-
Ɛ (max)
Dye 488: 83.000 cm-1M-1 | Dye 555: 91.000 cm-1M-1
-
Preparation/Handling
please see user manual of the kit
-
Shelf Life

