Pseudouridine Triphosphate (Pseudo-UTP)
Stabilizing Triphosphate for RNA
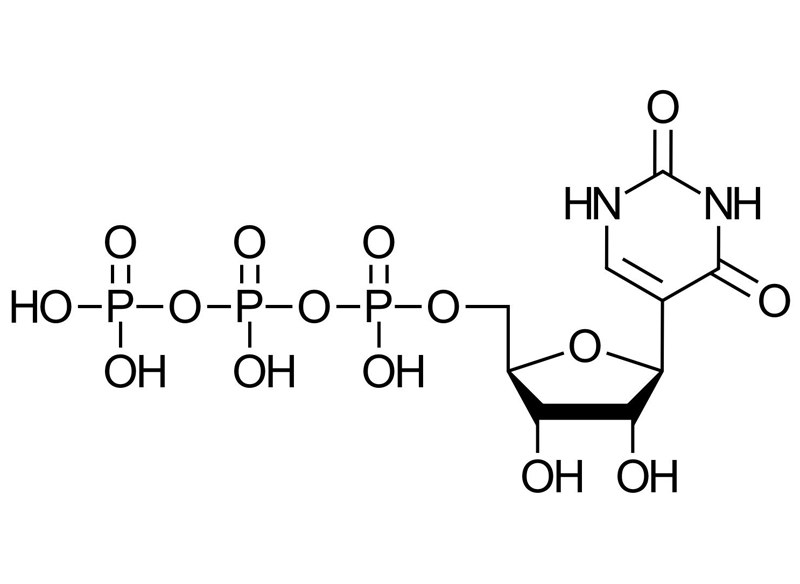
-
Pseudouridine Triphosphate (Pseudo-UTP) modified mRNA exhibits longer half-life, a better translation efficiency and its immunological properties are improved.
LITERATURE
Activation of Autoreactive B Cells by Endogenous TLR7 and TLR3 RNA Ligands, N. Green et al., 2012, J. Biol. Chem., Vol. 287(47), p. 39789-39799.
https://doi.org/10.1074/jbc.M112.383000
Transient Focal Membrane Deformation Induced by Arginine-rich Peptides Leads to Their Direct Penetration into Cells, H. Hirose et al., 2012, Mol Ther., Vol. 20(5), p. 984-993.
https://doi.org/10.1038/mt.2011.313
Feeder-Free Derivation of Human Induced Pluripotent Stem Cells with Messenger RNA, L. Warren et al., 2012, Sci Rep., Vol. 2, p. 657.
https://doi.org/10.1038/srep00657
Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L, B. Anderson et al., 2011, Nucleic Acids Research, Vol. 39(21), p. 9329-9338.
https://doi.org/10.1093/nar/gkr586
Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA, K. Karikó et al., 2011, Nucleic Acids Research, Vol. 39(21), p. e142.
https://doi.org/10.1093/nar/gkr695
Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation, B. Anderson et al., 2010, Nucleic Acids Research, Vol. 38(17), p. 5884-5892.
https://doi.org/10.1093/nar/gkq347
Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA, L. Warren et al., 2010, Cell Stem Cell, Vol. 7(5), p. 618-630.
https://doi.org/10.1016/j.stem.2010.08.012
Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability, K. Karikó et al., 2008, Molecular Therapy, Vol. 16(11), p. 1833-1840.
https://doi.org/10.1038/mt.2008.200
Norovirus Proteinase-Polymerase and Polymerase Are Both Active Forms of RNA-Dependent RNA Polymerase, G. Belliot et al., 2005, Journal of Virology, Vol. 79(4), p. 2393-2403.
https://doi.org/10.1128/jvi.79.4.2393-2403.2005
Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA, K. Karikó et al., 2005, Immunity, Vol. 23(2), p. 165-175.
https://doi.org/10.1016/j.immuni.2005.06.008
Pseudouridine in RNA: What, Where, How, and Why, M. Charette et al., 2000, IUBMB Life, Vol. 49(5), p. 341-351.
http://bpg.utoledo.edu/~afedorov/ABPG2011/L23/pseudouridine2000.pdf
Comparative Utilization of Pseudouridine Triphosphate and Uridine Triphosphate by Ribonucleic Acid Polymerase, I. Goldberg et al., 1963, J. Biol. Chem., Vol. 238(5), p. 1793-1800.
https://doi.org/10.1016/S0021-9258(18)81139-5
5-Ethynyluridine: A Bio-orthogonal Uridine Variant for mRNA-Based Therapies and Vaccines, S. Maassen et al., 2023, ChemBioChem, Vol. 24(5), e202200658.
https://doi.org/10.1002/cbic.202200658
Incorporation of Synthetic mRNA in Injectable Chitosan-Alginate Hybrid Hydrogels for Local and Sustained Expression of Exogenous Proteins in Cells, H. Steinle et al., 2018, Int. J. Mol. Sci., Vol. 19(5), p. 1313.
https://doi.org/10.3390/ijms19051313
Prediction of lipid nanoparticles for mRNA vaccines by the machine learning algorithm, W. Wang et al., 2022, Acta Pharmaceutica Sinica B, Vol. 12(6), p. 2950-2962.
-
-
Molecular Formula
C9H15N2O15P3
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C
-
Molecular Weight
484.14 g/mol
-
Purity
≥ 95% (HPLC)
-
Physical State
100 mM clear aquaeous solution; colorless
-
CAS Number
28022-82-4 (sodium salt)
1175-34-4 (free acid)
-
Additional name
5-Ribosyl Uracil, Pseudouridine-5′-triphosphate
-
Absorption (max)
λmax = 262 nm
-
Ɛ (max)
7,500 cm-1M-1
-
Molecular Formula

