THPTA | Tris(3-hydroxypropyltriazolylmethyl)amine
"baseclick-grade" water-soluble ligand in high quality for optimal yield
| Size | Catalog No. | Price |
|---|---|---|
| 10 mg | BCMI-006-10 | € 33,00 |
| 50 mg | BCMI-006-50 | € 70,00 |
| 100 mg | BCMI-006-100 | € 105,00 |
-
What is THPTA?
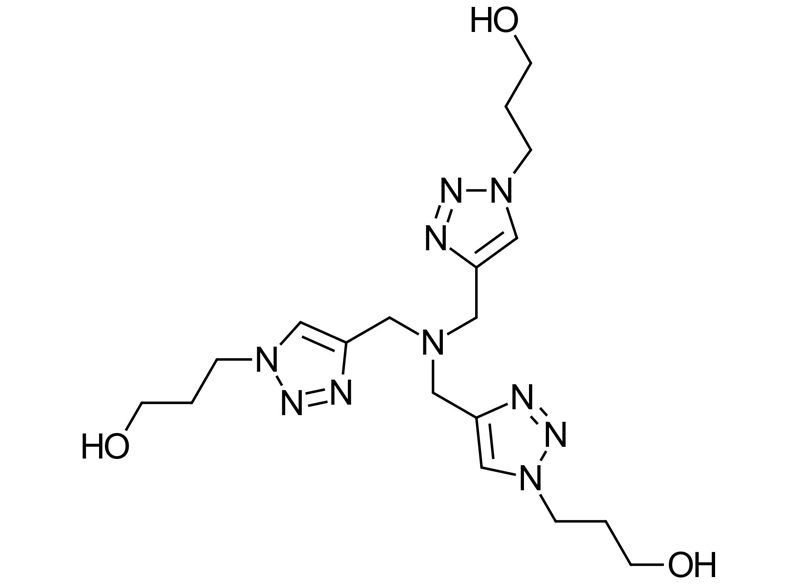
THPTA Tris(3-hydroxypropyltriazolylmethyl)amin is a tertiary amine that comprises three 1,2,3 triazole groups combined with 3 primary hydroxypropyl groups for enhanced water solubility. The THPTA ligand is a polytriazolylamine ligand, which is frequently used in coordination chemistry, namely in copper(I) catalyzed azide-alkyne cycloaddition (CuAAC). The copper ion Cu(I) will be stabilized in its +1 oxidation state by the THPTA ligand. Hereby, THPTA function is to protect it from oxidation and disproportionation. During this, THPTA can also increase the catalytic activity of the copper to increase the reaction rate of CuAAC. Due to THPTA’s stability under standard reaction conditions, THPTA ligand can be utilized as a versatile choice for many catalytic processes.
Chemical structure of THPTA
THPTA ligand is a symmetrical molecule consisting of a central tertiary amine connected to three identical substituents. All substituents are connected by a methylene group to the C4 of a 1,2,3-triazole. All triazoles have a hydroxypropyl group linked to the N1 of the triazole. These structures make THPTA to an ideal ligand for chelating copper ions in the biorthogonal CuAAC reaction. THPTA forms a cage-like complex with its 1,2,3-tetrazoles around the copper ion. Therefore, THPTA complex the copper ion and protects it from disproportion or oxidation by oxygen to increase its lifetime enabling a stable tolerant catalytic species for CuAAC reaction. The chelating of copper by THPTA ligand also reduces the likelihood of copper ions to catalyze the formation of reactive oxygen species which could lead to product degeneration. The hydroxypropyl groups of THPTA function as a polar group to increase the solubility of the THPTA in pure aqueous environments to enable Click Chemistry in biomolecules which may degrade in tertiary structure as proteins do in water solvent mixtures. baseclick is offering THPTA and other required materials to set up your own CuAAC reactions in high quality.
Areas of application of THPTA in research
DNA and RNA Modification with THPTA
THPTA ligand enables due to its high solubility in water to perform CuAAC reactions in pure aqueous solvent system and does not require water solvent mixtures. The possibility to use THPTA CuAAC in pure water is highly beneficial for click reactions with biomolecules that form specific tertiary and secondary structures that could be denatured when organic solvents as DMSO are added. Also, for click chemistry performed on RNA or DNA which tolerate water solvent mixtures THPTA provides several advantages as ligand in CuAAC as the fast reaction time about only 15-30 minutes.
The role of THPTA in Click-Chemistry
Studies comparing CuAAC reactions kinetics for reactions with and without THPTA found that the usage of THPTA ligand can increase reactions speed significantly. In combination with the potential of THPTA to prevent the formation of reactive oxygen species by copper-ions the usage of THPTA enables to perform CuAAC reactions in short time with high yields without degradation by the formation of reactive species. The increase of reaction kinetics allows for use of lower reaction temperatures which minimize the formation of side products while maintaining reasonable reaction times. The strong binding of THPTA to the copper ion also shields the copper ion for reaction with other reaction partners and therefore reduces its toxicity. All these facts make THPTA, or other ligands, an indispensable part of the CuAAC to be considered as click chemistry according to its definition.
THPTA-Enhanced Protein Coupling and Bioconjugation
The usage of the ligand THPTA in protein labeling by CuAAC is necessary because THPTA ligand stabilizes the catalytic effect Cu(I) oxidation state and meanwhile prevents the formation of reactive oxygen species (ROS), which are formed by copper species in THPTA ligand free environment. This ROS lead to oxidation of several amino acids as histidine, methionine or cysteine and therefore protein denaturation. Since THPTA can be used in completely aqueous environments, click reactions with THPTA can be performed without the need of the addition of organic solvents as DMSO. The addition of these organic solvents can cause changes in the secondary structure of proteins which is prevented by the usage of THPTA as ligand. Therefore, lots of protocols for labeling proteins with CuAAC in combination with THPTA have been published in the recent year highlighting its high yield and minimal protein degradation.
THPTA in Imaging Applications and Microscopy
THPTA enables to perform copper catalyzed azide alkyne cycloaddition (CuAAC) reaction with lower loads of the copper catalyst because of the elongation of the lifetime of the active Cu(I) catalyst. The reduced copper concentration when using THPTA ligand reduces the cytotoxic effects of copper. The shielding of copper by THPTA from catalyzing side reactions increases this effect additionally. THPTA also reduces the reaction time for CuAAC and therefore the time of copper exposure. Together these facts enable efficient fluorescent labeling of cell structures while causing minimal damage to the cell structure.
THPTA Applications in Drug Discovery and Pharmaceutical Research
THPTA is an essential part of CuAAC in aqueous phase when high yields, no side products and no product degradation should be achieved. These properties make CuAAC with THPTA a reaction considered to be click chemistry. These reactions are increasingly significant for the development of new drugs when there is the need to covalently ligate building blocks together. This technique is used to produce drugs as antibody drug conjugates (ADC) or to modify oligos to add structures that enable target delivery. In this field the use of THPTA supported CuAAC provides great advantages over the state-of-the-art linkage technique leading to safer products with lower cost due to higher coupling efficiency.
Practical advantages of THPTA in laboratory experiments
The specific form of the copper THPTA ligand complex forms a bulky structure where the triazole rings of THPTA function as a protective layer around the metal center of the complex shielding it from oxidating agents or other copper ions necessary for disproportionation. This structure enables the use of reduced copper concentrations since THPTA elongates the lifetime of the active copper (I) catalyst and leads to faster reaction. The binding of copper in the THPTA complex in combination with lower copper concentration necessary reduces the bioavailability of free copper ions significantly and therefore reduces toxicity for biological systems. The THPTA copper complex is also completely water soluble. This has the advantage of protecting biomolecular structures that are sensitive to organic solvents such as DMSO, which can alter their tertiary or secondary structure.
Future prospects and trends for THPTA
Emerging Research Fields for THPTA Applications
Next to THPTA use as ligand in CuAAC with all its benefits in improving labeling in biochemistry, production and modification of medicinal nanostructures or covalent linking of targeting structures to drugs as in ADC or siRNA delivery approaches, the THPTA copper complex can be used for selective cleavage of thiazolidines in proteins. The THPTA mediated release of a-oxo-aldehydes can be subsequently used for further protein modification on specific structures, which is a crucial step in the development of new protein or enzyme therapeutics. baseclick and ClickSeq have also developed a next generation sequencing kit based on THPTA supported CuAAC reaction for primer extension.
Innovation in THPTA Chemistry and Methodology
There are various attempts ongoing to modify THPTA ligand to optimize its properties to specific requests. Starting from simply modification of the chemical structure of the groups attached to the triazoles to modify the polarity of the ligand, which led to the development of ligands such as TBTA, BTTAA or BTTES. These similar ligands to THPTA have similar impact to CuAAC in terms of reaction kinetics and protection of the Cu(I) oxidation state but can have quite different properties in terms of solubility in certain solvent systems. There are also much further advanced chemicals changes to CuAAC ligands as the development of a covalent cage connecting the triazoles to form a molecular sphere around the central copper to shield it from any interfering nucleophile as glutathione. Further development of better complex ligand structures based on tertiary amines with tri-triazoles as used in THPTA have already enabled to perform CuAAC in living bacteria cells without destroying them.
LITERATURE
Copper-Catalyzed Azide–Alkyne Click Chemistry for Bioconjugation, S. Presolski et al., 2011, Current Protocols of Chemical Biology, Vol. 3(4), p. 153-162.
-
-
Molecular Formula
C18H30N10O3
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C
-
Molecular Weight
434.5 g/mol
-
Purity
≥ 95% (HPLC)
-
Physical State
white colored powder
-
CAS Number
760952-88-3
-
Additional name
Tris(3-hydroxypropyltriazolylmethyl)amine
-
Solubility
Water or DMSO/t-BuOH (Click Solution)
-
Molecular Formula

