
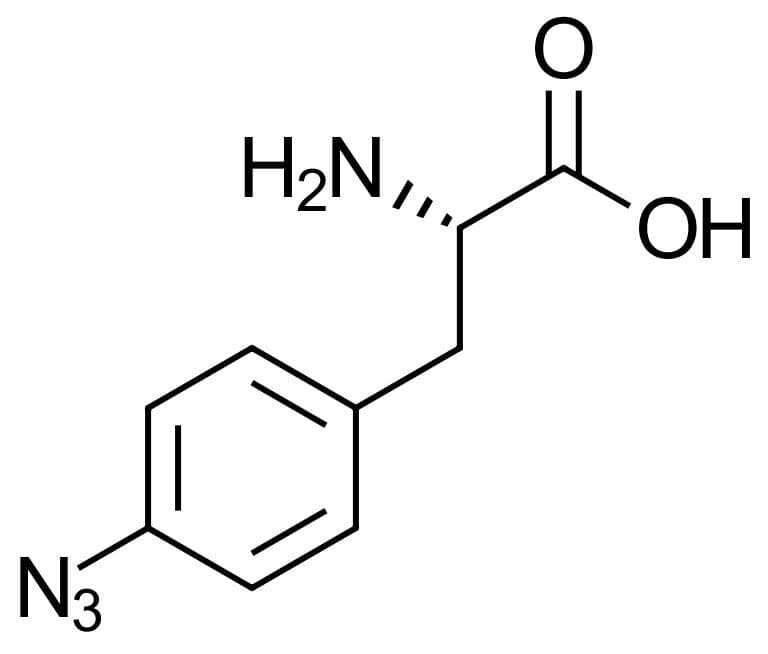
4-Azido-L-phenylalanine
Unnatural azido-modified amino acids for monitoring protein synthesis
| Size | Catalog No. | Price |
|---|---|---|
| 100 mg | BCAA-001-100 | € 150,00 |
Chemical Properties
-
Molecular Formula
C9H10N4O2
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dry
-
Molecular Weight
206.2 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
off-white to brownish powder
-
CAS Number
33173-53-4
-
Additional name
4-N3-L-phenylalanine; H-L-Phe(4-N3)-OH; H-L-4-azido-Phe-OH; H-Phe(4-N3)*HCl, H-4-Azido-Phe*HCl, Phe(pN3)*HCl, para-azido-Phe*HCl, 3-(p-azidophenyl)-L-alanine hydrochloride, 4-azidophenylalanine hydrochloride, p-azido-L-phenylalanine hydrochloride, p-azidophenylalanine hydrochloride
-
Solubility
c = 5% in AcOH 80% (clear slightly colored solution)
Product Information
Azido Amino Acid for Site‑Specific Protein Modification (Click Chemistry & Staudinger Ligation)
4‑Azido‑L‑phenylalanine (pAzF) is a synthetic azide‑bearing derivative of L‑phenylalanine used to introduce a bioorthogonal handle into proteins and peptides. It can be incorporated during translation via genetic code expansion (e.g., stop‑codon suppression at UAG with engineered tRNA/aaRS systems) or installed chemically by SPPS to yield azide‑functional peptides at defined positions. Once incorporated, the azido group undergoes copper‑free SPAAC with DBCO/BCN reagents or CuAAC with alkynes, forming stable triazoles for labeling, conjugation, or immobilization. Staudinger ligation is also possible for specialized workflows.
Use cases: precision protein/peptide labeling, antibody modification (including ADC strategies), glycan/ligand attachment (e.g., PEGs, dyes, sugars), and biomaterials interfaces, while maintaining native function under mild, biocompatible conditions.
Why 4‑Azido‑L‑phenylalanine?
Traditional NHS/thiol labeling is non‑selective and can over‑modify lysines/cysteines, often requiring basic pH and long incubations that risk structural changes. pAzF enables site‑specific, bioorthogonal coupling exactly at the engineered position, typically at neutral pH with short reaction times, reducing off‑target modifications and preserving tertiary structure.
Key Advantages
- Site Specificity by Design: Incorporate one defined azide per protein/peptide (genetic code expansion or SPPS) for precise positioning and consistent, reproducible conjugates.
- Mild, Fast Click Workflows: Perform SPAAC (DBCO/BCN) or CuAAC (alkyne) in aqueous buffers at near neutral pH; obtain stable triazoles with high to near quantitative conversions and straightforward purification.
- Structure Friendly Conditions: Bioorthogonal reactions at neutral pH help maintain folding and activity versus no selective NHS/thiol chemistries.
- Versatile Downstream Chemistry: Compatible with dyes, biotin, PEGs, sugars (e.g., GalNAc/Trimannose), affinity tags, and drug payloads for imaging, pull downs, targeting, and ADC like constructs.
- Cost Effective: High efficiency and clean conversions reduce waste and rework. Plus, baseclick offers competitive pricing across azido amino acids and DBCO partners.
Applications in Research & Industry
- Protein/Peptide Labeling (fluorophores, affinity tags)
- Antibody Modification (including steps in ADC development)
- Glycan/Targeting Conjugation (e.g., Tri‑/β‑GalNAc, mannose)
- Biomaterials/Surface Engineering (controlled immobilization)
- Proteomics & Mechanistic Studies (site‑specific probes)
LITERATURE
Cell-free co-production of an orthogonal transfer RNA activates efficient site-specific non-natural amino acid incorporation, C. Albayrak et al., 2013, Nucleic Acids Res., Vol. 41(11), p. 5949-5963.
https://doi.org/10.1093/nar/gkt226
An Expanded Eukaryotic Genetic Code, J. W. Chin et al., 2003, Science, Vol. 301(5635), p. 964-967.
https://www.science.org/doi/10.1126/science.1084772
Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome, H. Neumann et al., 2010, Nature, Vol. 464(7287), p. 441-444.
https://doi.org/10.1038/nature08817
The Locational Impact of Site-Specific PEGylation: Streamlined Screening with Cell-Free Protein Expression and Coarse-Grain Simulation, K. M. Wilding et al., 2018, ACS Synthetic Biology, Vol. 7(2), p. 510-521.
https://doi.org/10.1021/acssynbio.7b00316
Recoded organisms engineered to depend on synthetic amino acids, A. J. Rovner et al., 2015, Nature, Vol. 518(7537), p. 89-93.
https://doi.org/10.1038/nature14095
Uncovering the Domain-Specific Interactome of the TAF1 Tandem Reader Using Site-Specific Azide-Acetyllysine Photochemistry, Y. Yadav et al., 2023, Biochemistry, Vol. 62(2), p. 270–280.
https://doi.org/10.1021/acs.biochem.2c00140
Characterization and Scaled-Up Production of Azido-Functionalized Silk Fiber Produced by Transgenic Silkworms with an Expanded Genetic Code, H. Teramoto et al., 2019, Int. J. Mol. Sci., Vol. 20(3), p. 616.
https://doi.org/10.3390/ijms20030616
Genetic encoding of non-natural amino acids in Drosophila melanogaster Schneider 2 cells, T. Mukai et al., 2010, Protein Science, Vol. 19(3), p. 440-448.
https://doi.org/10.1002/pro.322
FAQ
-
How do I introduce 4‑Azido‑L‑phenylalanine into my protein?
Use genetic code expansion (engineered tRNA/aaRS recognizing UAG) for in‑cell incorporation, or employ SPPS to build azido‑modified peptides at precise positions.
-
Should I use SPAAC or CuAAC for labeling?
Choose SPAAC (DBCO/BCN) for copper‑free, highly biocompatible workflows (live cells, sensitive proteins). Use CuAAC with terminal alkynes when copper is acceptable and you want fast kinetics with standard catalysts/ligands.
-
What buffers/conditions work best?
For click steps, use neutral, oxygen‑tolerant buffers. For CuAAC, include a Cu(I) source and a stabilizing ligand (e.g., TBTA/THPTA) and appropriate reductant; for SPAAC, no catalyst is required.
-
Is pAzF compatible with antibodies and large complexes?
Yes, its site‑specific installation supports defined conjugation points, aiding control over DAR in ADC‑like constructs (subsequent payload attachment via SPAAC/CuAAC).
-
Any safety/handling notes?
Handle as a standard research chemical under good lab practice. Avoid unnecessary heating; store and use per SDS and product label. (For detailed guidance, consult the SDS.)
-
Is 4‑Azido‑L‑phenylalanine cost‑effective?
Yes. High labeling efficiency, short reaction times, and clean purifications reduce overall costs—and baseclick’s competitive pricing helps keep budgets in check (custom and bulk quotes available).

