
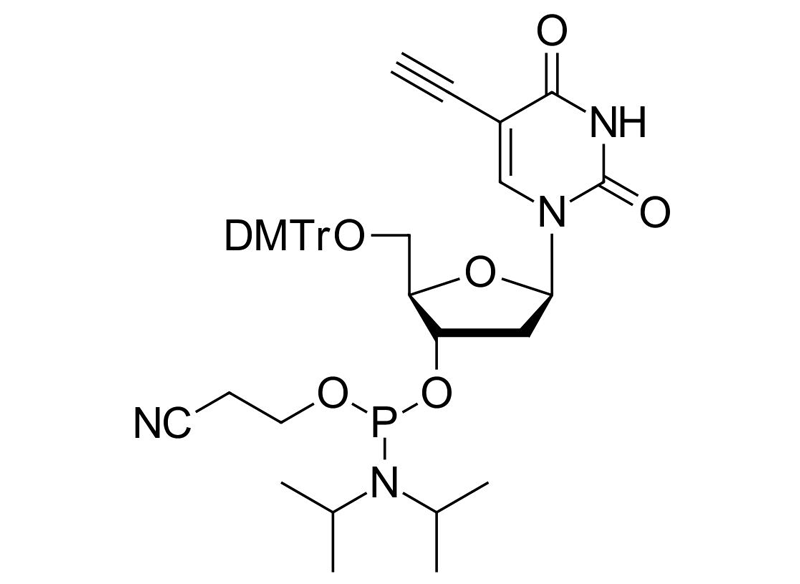
5-Ethynyl-dU-CE Phosphoramidite
Clickable phosphoramidite for Oligo synthesis
| Size | Catalog No. | Price |
|---|---|---|
| 250 mg | BCA-09-250 | € 320,00 |
| 1 g | BCA-09-1g | € 1.050,00 |
Chemical Properties
-
Molecular Formula
C41H47N4O8P
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C dry, inert gas
-
Molecular Weight
754.81 g/mol
-
Purity
≥ 95% (HPLC & 31P-NMR)
-
Physical State
white to light brown solid
-
CAS Number
615288-66-9
-
Additional name
5-Ethynyl-dU CEP; 2′-Deoxy-5′-DMT-5-ethynyluridine 3′-CE phosphoramidite; 5′-O-[Bis(4-methoxyphenyl)(phenyl)methyl]-3′-O-[(2-cyanoethoxy)(diisopropylamino)phosphino]-2′-deoxy-5-ethynyluridine; 5′-O-[Bis(4-methoxyphenyl)phenylmethyl]-2′-deoxy-5-ethynyl-uridine 3′-[2-cyanoethyl bis(1-methylethyl)phosphoramidite]
-
Preparation/Handling
This phosphoramidite is used under standard conditions for solid phase synthesis of oligonucleotides.
-
Solubility
MeCN
Product Information
Click-ready nucleotide analog for site-specific DNA labeling and functionalization
5-Ethynyl-dU-CE Phosphoramidite is a synthetic nucleotide analog derived from 5-ethynyl-2′-deoxyuridine (EdU), featuring an ethynyl (–C≡CH) group at the 5-position of the nucleobase uracil. This alkyne moiety enables bioorthogonal labeling via copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC), the foundation of click chemistry.
As a universal alkyne modifier, it integrates seamlessly into solid-phase DNA synthesis, offering high coupling efficiency and enabling site-specific functionalization for diverse applications from aptamer engineering to single-molecule sensing.
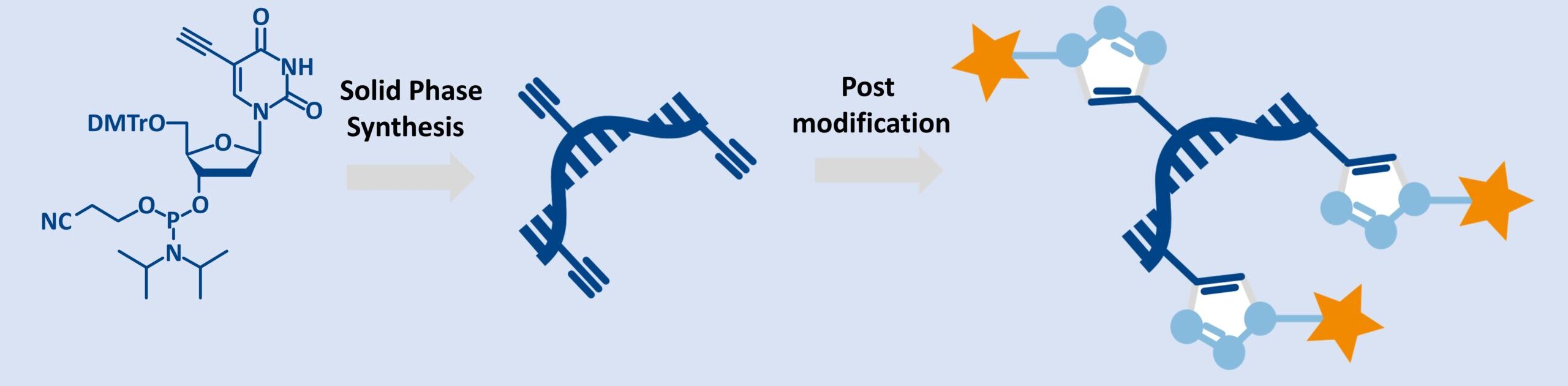
Before the introduction of 5-Ethynyl-dU-CE Phosphoramidite, DNA modification techniques were hampered by poor specificity, incompatibility with automated synthesis and biologically harsh reaction conditions.
This phosphoramidite solves these issues through smart design and clever chemistry:
- Click-ready alkyne: Enables selective conjugation with azides for labeling and bioconjugation.
- Solid-phase synthesis compatibility: Seamlessly fits into automated DNA synthesis workflows.
- Bioorthogonality: Chemically inert in biological systems until activated, allowing safe in vivo/in vitro use.
- Preserved DNA structure: The 5-position modification maintains Watson-Crick base pairing and duplex integrity
Applications
The versatility of 5-ethynyl-dU-CE-phosphoramidite extends over several areas:
- Fluorescent imaging and FRET: Enables high-resolution DNA visualization.
- Bioconjugation: Attaches DNA to proteins, nanoparticles, or surfaces.
- Diagnostics & therapeutics: Powers biosensors, hybridization probes, and modified oligonucleotide drugs.
- Cell proliferation assays: Related compounds like EdU revolutionized live-cell DNA synthesis tracking.
- Click-SELEX and aptamer engineering: Essential for generating clickmers aptamers with enhanced binding and chemical diversity.
- Programmable Nano-Reactors for Stochastic Sensing (PNRSS): In this cutting-edge platform, 5-Ethynyl-dU-CE enabled the creation of custom DNA strands with precisely placed alkyne handles.
5-Ethynyl-dU-CE Phosphoramidite is a cornerstone reagent in nucleic acid chemistry. By combining click chemistry compatibility, solid-phase synthesis efficiency, and protective strategies against hydration, it enables high-fidelity, modular DNA functionalization. Its role in aptamer development, molecular diagnostics, charge transport studies, and programmable nano-reactors [1] continue to drive innovation in synthetic biology, nanotechnology, and chemical biology.
[1] Programmable nano-reactors for stochastic sensing, S. Huang et al., Nat. Commun. 2021, 12, 5811.
LITERATURE
DNA duplexes stabilized by modified monomer residues: synthesis and stability, D. Graham et al., 1998, J. Chem. Soc., Perkin Trans. 1, p. 1131–1138.
https://doi.org/10.1039/A707031D
A Versatile Modification of On-Column Oligodeoxynucleotides Using a Copper-Catalyzed Oxidative Acetylenic Coupling Reaction, N. Minakawa et al., 2003, J. Am. Chem. Soc., Vol. 125, p. 11545–11552.
https://doi.org/10.1021/ja036055t
Ethynyl Side Chain Hydration during Synthesis and Workup of “Clickable” Oligonucleotides: Bypassing Acetyl Group Formation by Triisopropylsilyl Protection, S. A. Ingale et al., 2013, J. Org. Chem., Vol. 78, p. 11271–11282.
https://doi.org/10.1021/jo401780u
Click Reaction on Solid Phase Enables High Fidelity Synthesis of Nucleobase-Modified DNA, F. Tolle et al., 2016, Bioconjugate Chem., Vol. 27, p. 500–503.
https://doi.org/10.1021/acs.bioconjchem.5b00668
Efficient Long-Range Hole Transport Through G-Quadruplexes, J. Wu et al., 2017, Chemistry – A European Journal, Vol. 23(56), p. 13980-13985.
https://doi.org/10.1002/chem.201702478
Pyrene-Modified DNA Aptamers with High Affinity to Wild-Type EGFR and EGFRvIII, E. Zavyalova et al., 2020, Nucleic Acid Therapeutics, Vol. 30(3), p. 175-187.

