
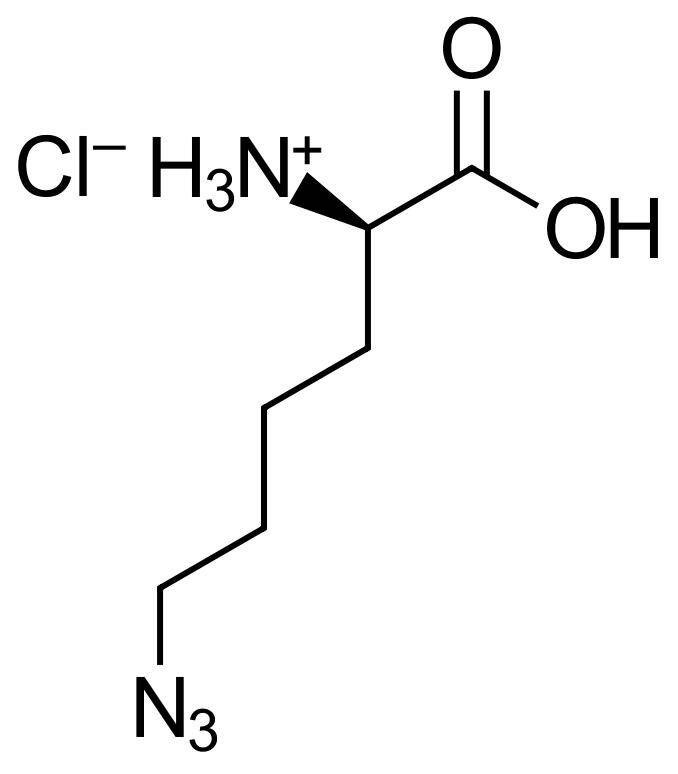
6-Azido-D-lysine HCl
Azido-modified amino acids for bioconjugation with alkyne reporters
| Size | Catalog No. | Price |
|---|---|---|
| 100 mg | BCAA-010-100 | € 140,00 |
Chemical Properties
-
Molecular Formula
C6H12N4O2 *HCI
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
2-8 °C, dry
-
Molecular Weight
172.19 g/mol * 36.45 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
white crystalline powder
-
CAS Number
2098497-01-7 (hydrochloride salt)
1418009-93-4 (free acid) -
Additional name
H-D-Lys(N3)-OH*HCl; N-epsilon-Azido-D-lysine; (R)-2-Amino-6-azidohexanoic acid hydrochloride
Product Information
Stable and Bioorthogonal Protein Labeling with D-Amino Acid Technology
6-Azido-D-lysine ((R)-2-Amino-6-azidohexanoic acid hydrochloride) is a synthetic unnatural D-amino acid derivative of lysine carrying an azide group. This reagent enables site-specific protein modification and bioorthogonal labeling via Click Chemistry (SPAAC or CuAAC) or Staudinger ligation under mild conditions.
Incorporation Methods
- Genetic Code Expansion: Engineered tRNAs (based on prokaryotic tRNAs) recognize amber codons (usually UAG) and incorporate 6-Azido-D-lysine at a defined position during biosynthesis[1].
- Solid Phase Peptide Synthesis (SPPS): Allows chemical incorporation at any position in the peptide sequence for versatile functionalization.
After incorporation, the azide moiety reacts selectively with alkyne-modified partners (DBCO, BCN, PEGs, dyes) or P(III)-containing reagents, enabling efficient conjugation without side reactions.
Why choose 6-Azido-D-lysine?
Traditional NHS ester chemistry reacts with primary amines (e.g., lysines), leading to:
- Non-specific labeling at multiple sites
- Requires basic pH (8–9) and overnight reactions
- Risks protein degradation and structural changes
In contrast, azide-based labeling with 6-Azido-D-lysine offers:
- Site-specific modification at the planned position
- Fast reactions at neutral pH with high yields
- Preserves tertiary structure
- Fully biorthogonal, no interference with native functional groups
- Lower reagent consumption and atom-economic click chemistry for cost-effective, eco-friendly workflows
- Unique Benefits of D-Amino Acid Incorporation
Unique Benefits of D-Amino Acid Incorporation
The usage of a D-amino acid instead of an L-amino azide for introduction of the labels has several influences on the peptide.
- Increased stability: Peptides containing a D-amino acid are more stable than only L-amino acids containing peptides because proteases and peptidases can not break a bond involving a D-amino acid.
- Structural Variations: D-amino acids alter peptide folding (e.g., prevent α-helix formation), enabling novel structural designs for research and drug development.
Key Advantages of 6-Azido-D-lysine:
- Integrity of tertiary structure: SPAAC and CuAAC reactions can be performed at neutral pH and in short reaction times. Therefore, the chemical modification of your peptide/protein is possible without the risk of the target biomolecule degrading.
- Fast & Efficient Click Reactions: The used click chemistry forms highly stable triazole bonds with all alkynes or alkyne derivates which can be used for strain-promoted alkyne-azide cycloaddition in short reaction time.
- Specific labeling: Since 4-Azido-L-homoalanine is incorporated into your peptide/protein at a precisely defined position, highly specific labeling at this position is possible.
- Increased stability: Peptides including D-amino acids are more stable against enzymatic degradation.
This reagent is used for:
- Protein/Peptide labeling
- Solid phase peptide synthesis (SPPS)
- Antibody labeling
- Antibody modification for the synthesis of antibody-drug conjugates (ADCs)
- Linking targeting moieties to peptides/proteins
LITERATURE
[1] Incorporating unnatural amino acids into recombinant proteins in living cells, Mitra, N., 2013, Materials and Methods, 3(204), 1.
https://doi.org/10.13070/mm.en.3.204
Synthesis of biologically active nickelocenyl–amino acid conjugates using 1,3-dipolar cycloaddition click reactions, M. A. Raza et al., 2017, Russ. J. Gen. Chem., Vol. 87, p. 2678–2683.
https://doi.org/10.1134/S107036321711024X
Chemically Induced Cell Wall Stapling in Bacteria, S. L. Rivera et al., 2021, Cell Chemical Biology, Vol. 28(2), p. 213-220.
https://doi.org/10.1016/j.chembiol.2020.11.006
Systematic Assessment of Accessibility to the Surface of Staphylococcus aureus, N. J. Ferraro et al., 2021, ACS Chem. Biol., Vol. 16(11), p. 2527–2536.
https://doi.org/10.1021/acschembio.1c00604
FAQ
-
Is it possible to generate azide- or alkyne-modified peptides?
With modified amino acids azide- or alkyne-modified peptides can be prepared by solid-phase synthesis.
-
How can de novo protein biosynthesis be monitored?
De novo protein biosynthesis can be monitored by feeding of metabolite analogues (so-called metabolic labeling) and subsequent click reaction. Azido-homoalanine for example is recognized as a methionine analogue and is incorporated into de novo synthesized proteins in methionine-free medium conditions. The resulting proteins contain azide moieties and thus can be detected after click to an alkyne-containing reporter molecule (e.g. a fluorescent dye). This non-radioactive method has major practical advantages compared to traditional 35S amino acid incorporation methods.
Alternatively, O-propargyl-puromycin is efficiently incorporated into proteins during de novo protein biosynthesis and can be used in complete medium. The resulting alkyne protein fragments can be detected via click to azide-containing reporter molecules. -
Why use 6-Azido-D-lysine instead of traditional NHS ester chemistry?
Unlike NHS ester labeling, which is non-specific and requires harsh conditions, 6-Azido-D-lysine enables site-specific labeling under mild, neutral pH conditions. This preserves protein structure and avoids multiple modification sites.
-
Which click reactions are supported?
Both SPAAC (strain-promoted) and CuAAC (copper-catalyzed) reactions are compatible. SPAAC is ideal for live-cell applications due to its copper-free nature, while CuAAC offers rapid and efficient conjugation in vitro.
-
What is the advantage of using a D-amino acid?
D-amino acids increase peptide stability by making them resistant to enzymatic degradation and can alter tertiary structure, enabling novel designs for research and drug development.
-
Can 6-Azido-D-lysine be used in solid-phase peptide synthesis (SPPS)?
Yes. It can be incorporated at any position during SPPS, allowing versatile functionalization for custom peptide design.
-
What labeling partners are compatible?
Common partners include DBCO/BCN-modified PEGs, fluorescent dyes, antibodies, and targeting moieties for advanced bioconjugation workflows.
-
Is the reaction bioorthogonal?
Yes. Click chemistry with azides is fully bioorthogonal, meaning no side reactions occur with native functional groups in peptides or proteins.

