
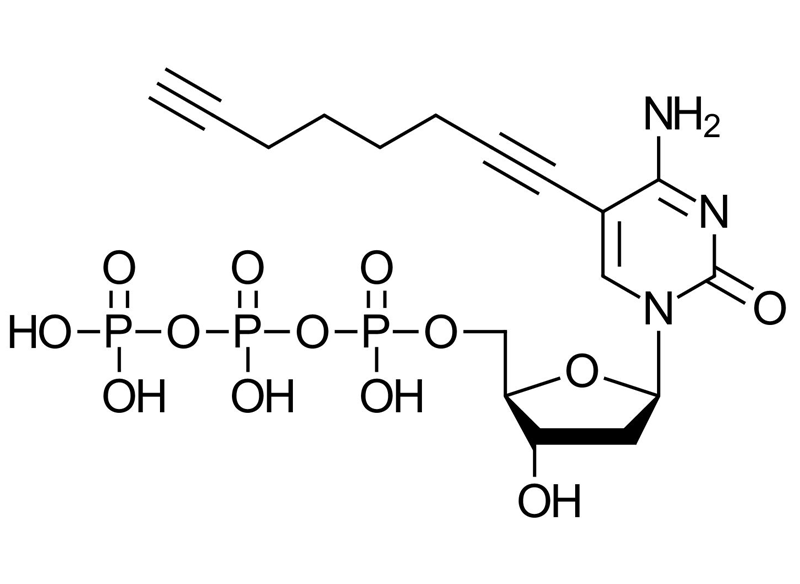
C8-Alkyne-dCTP
Modified triphosphate for incorporation in PCR reaction
| Size | Catalog No. | Price |
|---|---|---|
| 1 µmol | BCT-06-S | € 100,00 |
| 5 µmol | BCT-06-L | € 300,00 |
Chemical Properties
-
Molecular Formula
C17H24N3O13P3
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C
-
Molecular Weight
571.31 g/mol
-
Purity
≥ 95% (HPLC)
-
Physical State
100 mM solution in water (pH 7.5); clear colorless to light yellow solution
-
CAS Number
1374991-80-6 (sodium salt)
1004297-66-8 (free acid)
-
Absorption (max)
λmax = 295 nm
-
Ɛ (max)
9,500 cm-1M-1
Product Information
High-Purity Modified dCTP for Precision DNA Labeling via Click Chemistry
C8-Alkyne-dCTP is a chemically engineered deoxycytidine triphosphate (dCTP) featuring an alkyne moiety at the C8 position of cytosine. This strategic modification enables bioorthogonal conjugation via Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC), making it a powerful tool for site-specific DNA labeling, molecular tracking, and functionalization during enzymatic synthesis. Unlike conventional labeling reagents, C8-Alkyne-dCTP integrates directly into DNA strands during synthesis, streamlining workflows and enhancing reproducibility. Its compatibility with widely used DNA polymerases and mild reaction conditions.
Why Buy C8-Alkyne-dCTP?
DNA modification has long faced challenges:
- Natural nucleotides lack reactive chemical groups, limiting functionalization.
- DNA polymerases often reject modified nucleotides or incorporate them inefficiently.
- Earlier labeling methods were non-specific and lacked control over modification sites.
- Traditional conjugation techniques required harsh conditions that could damage DNA or disrupt biological systems.
C8-Alkyne-dCTP overcomes these limitations with several key advantages:
- Bioorthogonal Reactivity: The alkyne group enables highly selective reactions with azide-containing molecules via CuAAC. This reaction is efficient, specific, and occurs under mild, aqueous conditions, preserving DNA integrity.
- Enhanced Polymerase Compatibility: Compatible with family B polymerases (Pwo, Deep Vent exo-, KOD XL) and family A polymerase Taq, ensuring smooth and efficient incorporation into DNA strands.
- Site-Specific Functionalization: Researchers can precisely control nucleotide incorporation, enabling targeted modification of DNA sequences for high-resolution applications.
- Physiological Reaction Conditions: Click chemistry is aqueous and biologically compatible, allowing safe and reliable DNA modification in sensitive environments.
- Safe dNTP Quantification & Chemotherapeutic Monitoring: Replaces traditional radioisotope-based detection with fluorophore-based assays, improving safety, throughput, and reproducibility in cancer research and drug monitoring workflows.
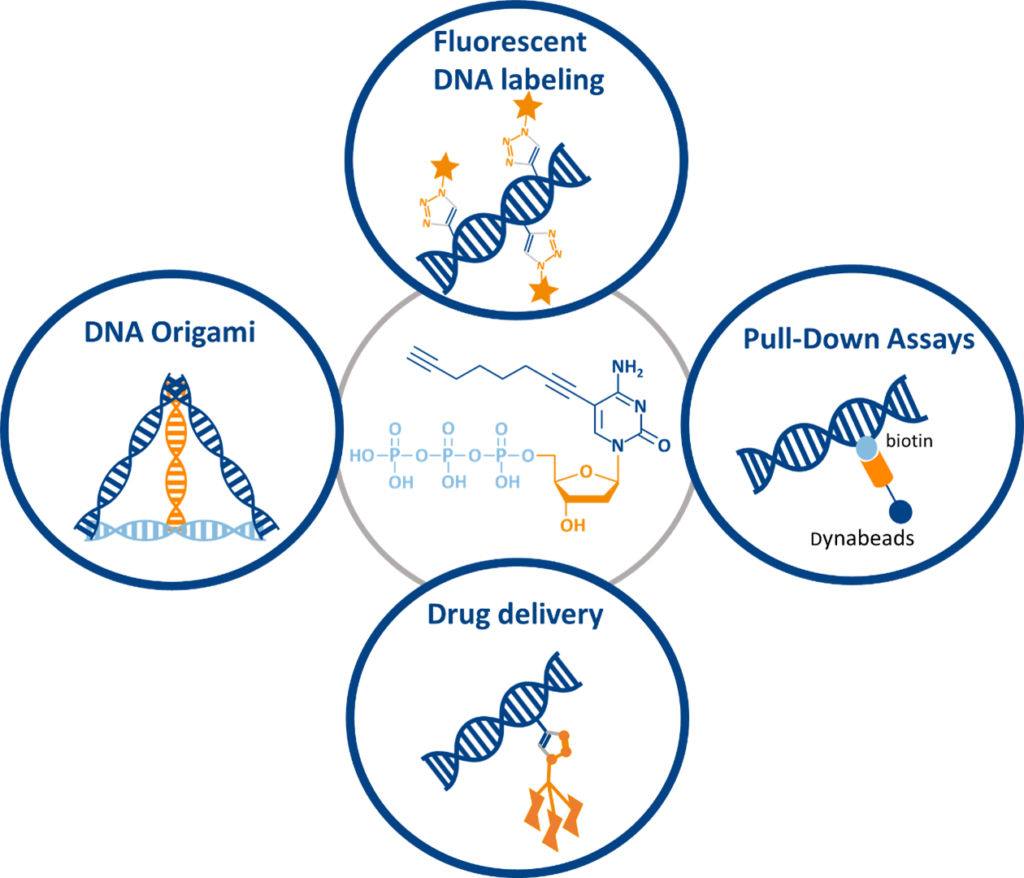
Applications of C8-Alkyne-dCTP
C8-Alkyne-dCTP is a versatile reagent used across multiple research and diagnostic fields:
- Fluorescent DNA Labeling
Enables high-resolution microscopic imaging and real-time tracking of DNA interactions. - Pull-Down Assays
Facilitates biotin-azide conjugation for identifying DNA-binding proteins and studying protein-DNA interactions. - DNA Origami & Nanostructure Functionalization
Supports nanotechnology applications, including DNA metallization for nanoelectronics and biosensors. - Targeted Drug Delivery & Molecular Diagnostics
Allows precise DNA modifications for therapeutic targeting, biomarker detection, and custom assay development.
LITERATURE
Synthesis of Highly Modified DNA by a Combination of PCR with Alkyne-Bearing Triphosphates and Click Chemistry, J. Gierlich et al., 2007, Chem. – A Eur. J., Vol. 13, p. 9486–9494.
https://doi.org/10.1002/chem.200700502
Directed DNA Metallization, G. A. Burley et al., 2006, J. Am. Chem. Soc., Vol. 128, p. 1398–1399.
https://doi.org/10.1021/ja055517v
Quantitation of deoxynucleoside triphosphates by click reactions, C. Y. Huang et al., 2020, Scientific Reports, Vol. 10(1), p. 611.

