
DBCO-C6-Alkyne
Bifunctional linker
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCL-035-5 | € 110,00 |
| 10 mg | BCL-035-10 | € 180,00 |
Chemical Properties
-
Molecular Formula
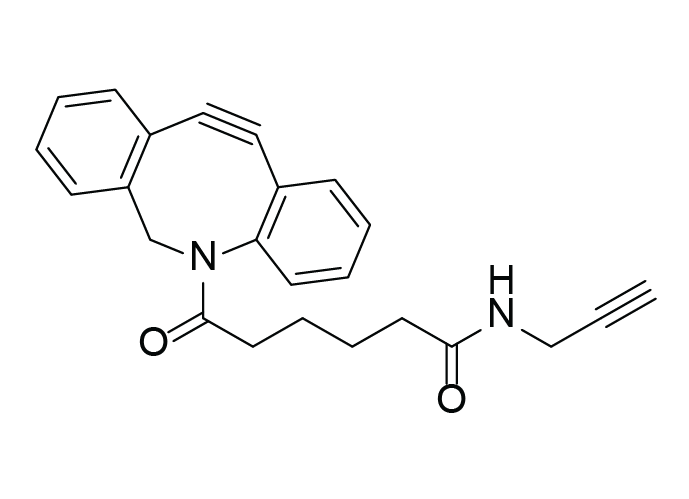
C24H22N2O2
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark
-
Molecular Weight
370.45 g/mol
-
Purity
≥ 95% (HPLC)
-
Physical State
white to off-white solid
-
CAS Number
n.a.
-
Additional name
N-(propargylamidoadipoyl)-dibenzoazacyclooctyne
-
Solubility
DMSO, DMF, DCM, THF, Chloroform
-
Preparation/Handling
For a 10 mM solution add 1350 μL to 5 mg.
For a 10 mM solution add 2699 μL to 10 mg.
Product Information
Heterobifunctional Linker for Sequential Click Chemistry Conjugation
DBCO‑C6‑Alkyne (N‑(propargylamidoadipoyl)‑dibenzoazacyclooctyne) is a heterobifunctional crosslinker combining two orthogonal reactive groups:
- DBCO for copper‑free SPAAC (strain‑promoted azide–alkyne cycloaddition)
- Terminal alkyne for CuAAC (copper‑catalyzed azide–alkyne cycloaddition)
This design enables two sequential click reactions under mild, bioorthogonal conditions, allowing precise molecular assembly without compromising biomolecule integrity. The DBCO group reacts rapidly with azides in the absence of copper, while the terminal alkyne can subsequently undergo CuAAC with another azide in the presence of a copper catalyst or vice versa if required[1].
Why DBCO‑C6‑Alkyne?
Traditional conjugation methods (e.g., NHS‑ester chemistry) often require harsh conditions, exhibit poor selectivity, and risk damaging sensitive biomolecules. DBCO-PEG4-Alkyne overcomes these limitations by leveraging the 2022 Nobel Prize‑winning click chemistry principles for highly selective, efficient, and biocompatible crosslinking.
Key Advantages
- Green Chemistry Approach: Click reactions are atom‑economic, require non‑toxic solvents, and generate minimal waste—making them environmentally friendly and cost‑effective.
- Fast & Efficient: SPAAC and CuAAC form stable triazole linkages quickly and with high yield.
- Orthogonal Reactivity: Enables two distinct click reactions in a controlled sequence for advanced molecular engineering.
- Biocompatibility: Mild conditions preserve biomolecule structure and function.
For highly polar environments (e.g., aqueous buffers), we recommend DBCO‑PEG4‑Alkyne, which offers improved solubility while maintaining the same functional groups.
Applications
- Antibody–Drug Conjugates (ADCs): Site‑specific dual labeling for targeted therapeutics.
- Crosslinking of Azido‑Modified Molecules: Proteins, peptides, nucleic acids, and polymers.
- Targeted Drug Delivery: Construction of multifunctional conjugates for precision medicine.
LITERATURE
[1] Transient protection of strained alkynes from click reaction via complexation with copper, S. Yoshida et al., 2014, Journal of the American Chemical Society, Vol. 136(39), p. 13590-13593.

