
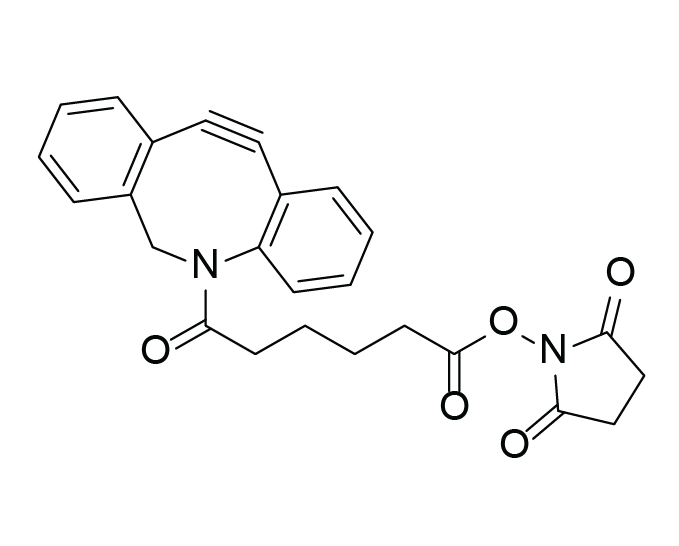
DBCO-C6-NHS ester
Bifunctional linker
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCL-037-5 | € 60,00 |
| 10 mg | BCL-037-10 | € 90,00 |
| 50 mg | BCL-037-50 | € 240,00 |
Chemical Properties
-
Molecular Formula
C25H22N2O5
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark
-
Molecular Weight
430.5 g/mol
-
Purity
≥ 95% (HPLC)
-
Physical State
white to slightly grey crystalline solid
-
CAS Number
1384870-47-6
-
Additional name
1-{[6-(11,12-didehydrodibenzo[b,f]azocin-5(6H)-yl)-6-oxohexanoyl]oxy}-2,5-pyrrolidinedione
-
Solubility
DCM, THF, Acetonitrile, DMF, DMSO
-
Preparation/Handling
For a 10 mM solution add 1161 μL to 5 mg.
For a 10 mM solution add 2323 μL to 10 mg.
Product Information
Heterobifunctional Linker for Amine Coupling and Copper‑Free Click Chemistry
DBCO‑C6‑NHS Ester is a heterobifunctional crosslinker combining:
- NHS ester for selective coupling to primary amines
- DBCO for copper‑free SPAAC (strain‑promoted azide–alkyne cycloaddition)
This dual functionality enables orthogonal bioconjugation under mild conditions, making it ideal for introducing DBCO groups to amine‑containing biomolecules such as proteins, peptides, antibodies, and amine‑modified oligonucleotides. Once installed, the DBCO moiety reacts rapidly with azides without the need for copper catalysts, forming stable triazole linkages.
Why DBCO‑C6‑NHS Ester?
Traditional conjugation methods often require harsh conditions, exhibit poor selectivity and risk damaging sensitive biomolecules. DBCO‑C6‑NHS Ester provides a biocompatible, efficient, and stable platform for precise molecular engineering in pharmaceutical development, diagnostics, and advanced biotechnology.
Key Advantages
- Dual Reactivity: Introduces a DBCO group to primary amine‑containing biomolecules, enabling subsequent click chemistry. The C6 spacer minimizes steric hindrance and improves accessibility.
Example: Post‑synthetic DBCO labeling of amino‑modified oligonucleotides, where DBCO cannot survive solid‑phase synthesis conditions. - Fast & Efficient Click Reactions: Forms stable triazole bonds with azides under catalyst‑free conditions, achieving high to quantitative yields.
- Compatibility with Organic Media: Soluble in nonpolar solvents (e.g., DCM, THF, ethyl acetate), making it suitable for organic synthesis, surface functionalization and hydrophobic conjugates. For biomolecules incompatible with organic solvents, use DBCO‑PEG4‑NHS Ester for improved polarity and aqueous compatibility.
Applications in Research and industry
- Antibody–Drug Conjugates (ADCs): Site‑specific functionalization for targeted therapeutics.
- Surface and Hydrogel Modifications: Controlled immobilization of biomolecules.
- Aptamer and Oligonucleotide Functionalization: Post‑synthetic DBCO introduction for click labeling.
- Organic Synthesis: Efficient crosslinking in nonpolar environments.
Literature
ICAM-1-targeted and antibacterial peptide modified polymeric nanoparticles for specific combating sepsis, L. Pan et al., 2022, Materials & Design, Vol. 222, 111007.
https://doi.org/10.1016/j.matdes.2022.111007
Preparation of single- and double-oligonucleotide antibody conjugates and their application for protein analytics, J. Wiener et al., 2020, Scientific Reports, Vol. 10(1), 1457.
https://doi.org/10.1038/s41598-020-58238-6
SMAP3-ID for Identification of Endogenous Protein–Protein Interactions Reveals Regulation of Mitochondrial Activity by Lamins, J.Warren et al., 2025, JACS Au, 5(1), 302-319.

