
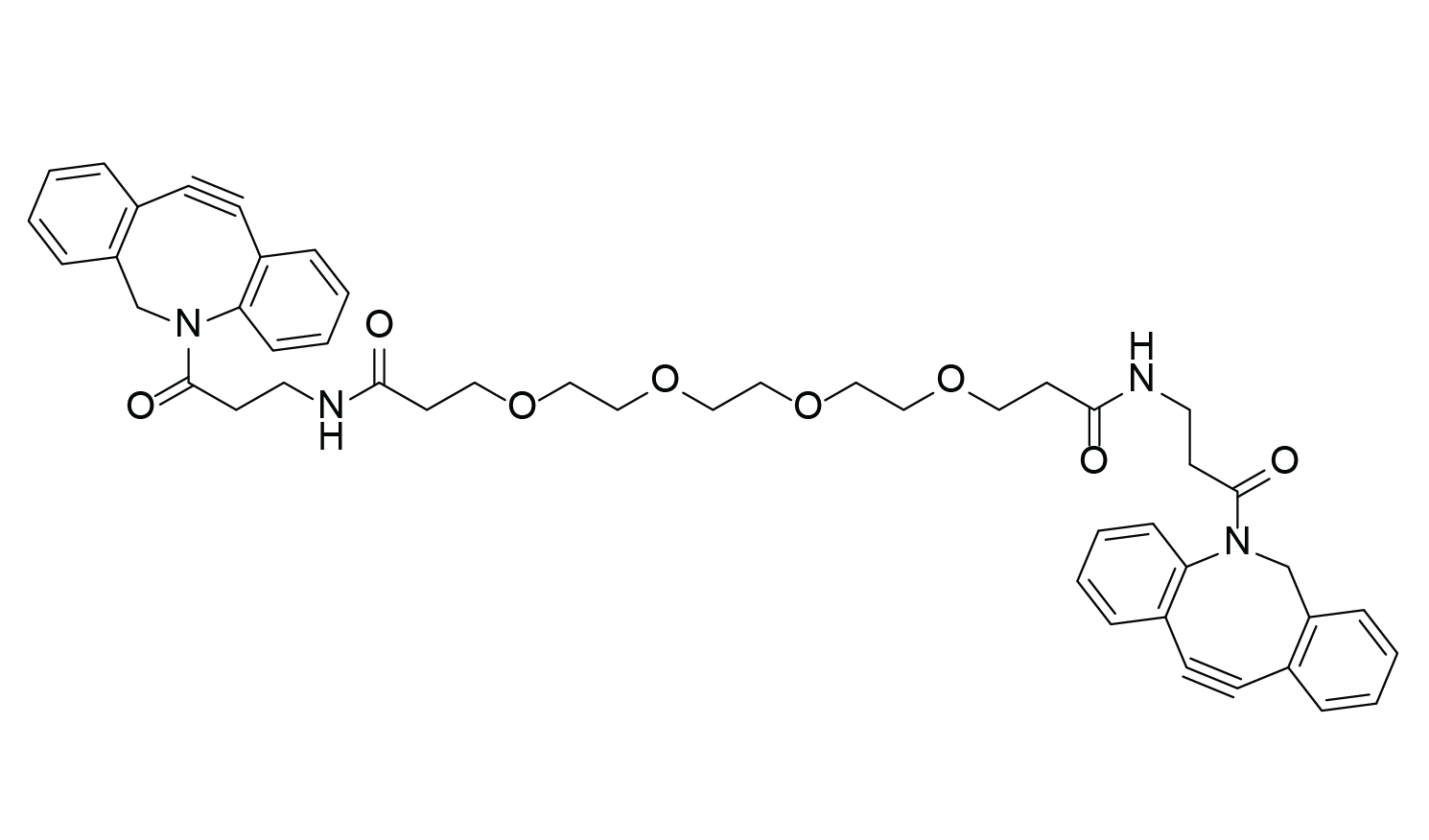
DBCO-PEG4-DBCO
Homobifunctional linker
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCL-039-5 | € 100,00 |
| 10 mg | BCL-039-10 | € 150,00 |
Chemical Properties
-
Molecular Formula
C48H50N4O8
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark, dry
-
Molecular Weight
811 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
yellow gel
-
CAS Number
2182601-68-7
-
Solubility
DMSO, MeOH, DMF
-
Preparation/Handling
For a 10 mM solution add 123 μL per 1 mg.
Product Information
Homobifunctional Linker for Copper‑Free Click Chemistry
DBCO‑PEG4‑DBCO is a homobifunctional crosslinker combining:
• Two DBCO groups for copper‑free SPAAC (strain‑promoted azide–alkyne cycloaddition)
• A PEG4 spacer for improved solubility and reduced steric hindrance
This design enables selective, biocompatible crosslinking and nucleic acid circularization under mild conditions. Both DBCO moieties react rapidly with azides without the need for copper catalysts, forming stable triazole linkages.
Why DBCO‑PEG4‑DBCO?
Traditional conjugation methods often require harsh conditions, exhibit poor selectivity, and risk damaging sensitive biomolecules. DBCO‑PEG4‑DBCO provides a highly efficient and stable platform for precise molecular engineering in pharmaceutical development, diagnostics and advanced biotechnology.
Key Advantages
- Homobifunctional Reactivity: Two DBCO groups enable dual azide coupling for crosslinking or circularization. The PEG4 spacer improves aqueous compatibility and minimizes steric hindrance.
- Fast & Efficient Click Reactions: Forms stable triazole bonds with azides under catalyst‑free conditions, achieving high to quantitative yields.
- Green Chemistry: SPAAC is atom‑economic, copper‑free, and easy to purify — reducing waste and environmental impact compared to CuAAC.
Applications in Research and Industry
- Antibody–Drug Conjugates (ADCs): Controlled crosslinking for targeted therapeutics
- Surface and Hydrogel Modifications: Functionalization for biomaterials and biosensors
- Nucleic Acid Circularization: Efficient assembly of DNA/RNA constructs
Comparison of baseclick DBCO Linkers for SPAAC click
| Feature / Property | DBCO‑PEG4‑DBCO | DBCO‑PEG4‑NHS Ester | DBCO‑C6‑NHS Ester |
| Type | Homobifunctional linker | Heterobifunctional linker | Heterobifunctional linker |
| Functional Groups | 2 × DBCO | DBCO + NHS ester | DBCO + NHS ester |
| Spacer | PEG4 (hydrophilic) | PEG4 (hydrophilic) | C6 (hydrophobic) |
| Primary Use | Crosslinking, nucleic acid circularization | Introduce DBCO to amine‑containing biomolecules | Introduce DBCO to amine‑containing biomolecules |
| Solubility | Aqueous compatible | Aqueous compatible | Organic solvents (DCM, THF, EtOAc) |
| Click Reaction | SPAAC (copper‑free) | SPAAC (copper‑free) | SPAAC (copper‑free) |
| Ideal For | Dual azide coupling, precise crosslinking | Proteins, peptides, oligos (amine modification) | Organic synthesis, hydrophobic conjugates |
| Key Advantage | Enables two‑step conjugation for defined products | Adds DBCO to biomolecules under mild conditions | Suitable for nonpolar environments |
Literature
Generation of therapeutic protein variants with the human serum albumin binding capacity via site-specific fatty acid conjugation, J. Cho et al., 2017, Scientific Reports, Vol. 7(1), 18041.
https://doi.org/10.1038/s41598-017-18029-y
An expanded genetic code facilitates antibody chemical conjugation involving the lambda light chain, A. Kato et al., 2021, Biochemical and Biophysical Research Communications, Vol. 546, p. 35-39.
https://doi.org/10.1016/j.bbrc.2021.02.005
Characterization and scaled-up production of azido-functionalized silk fiber produced by transgenic silkworms with an expanded genetic code, H. Teramoto et al., 2019, International Journal of Molecular Sciences, Vol. 20(3), 616.
https://doi.org/10.3390/ijms20030616
Orthogonal End Labelling of Oligonucleotides through Dual Incorporation of Click‐Reactive NTP Analogues, E. Schönegger et al., 2023. ChemBioChem, 25(1), e202300701.
https://doi.org/10.1002/cbic.202300701
FAQ
-
Does DBCO-PEG4-DBCO require copper?
No. It is designed for copper‑free, strain‑promoted click reactions (SPAAC), ensuring high biocompatibility for sensitive biomolecules.
-
How do I avoid forming “double adducts”?
Use DBCO‑PEG4‑DBCO in molar excess for the first coupling, purify, then add the second azide. This two‑step approach yields defined single‑adduct intermediates and clean final products.
-
Why is the PEG4 spacer useful?
It improves aqueous compatibility, provides distance between conjugated partners to reduce steric clashes, and can mitigate aggregation in complex systems.
-
What are typical use cases?
ADCs, surface/hydrogel modification, and circularization of nucleic acids in research and development workflows.

