
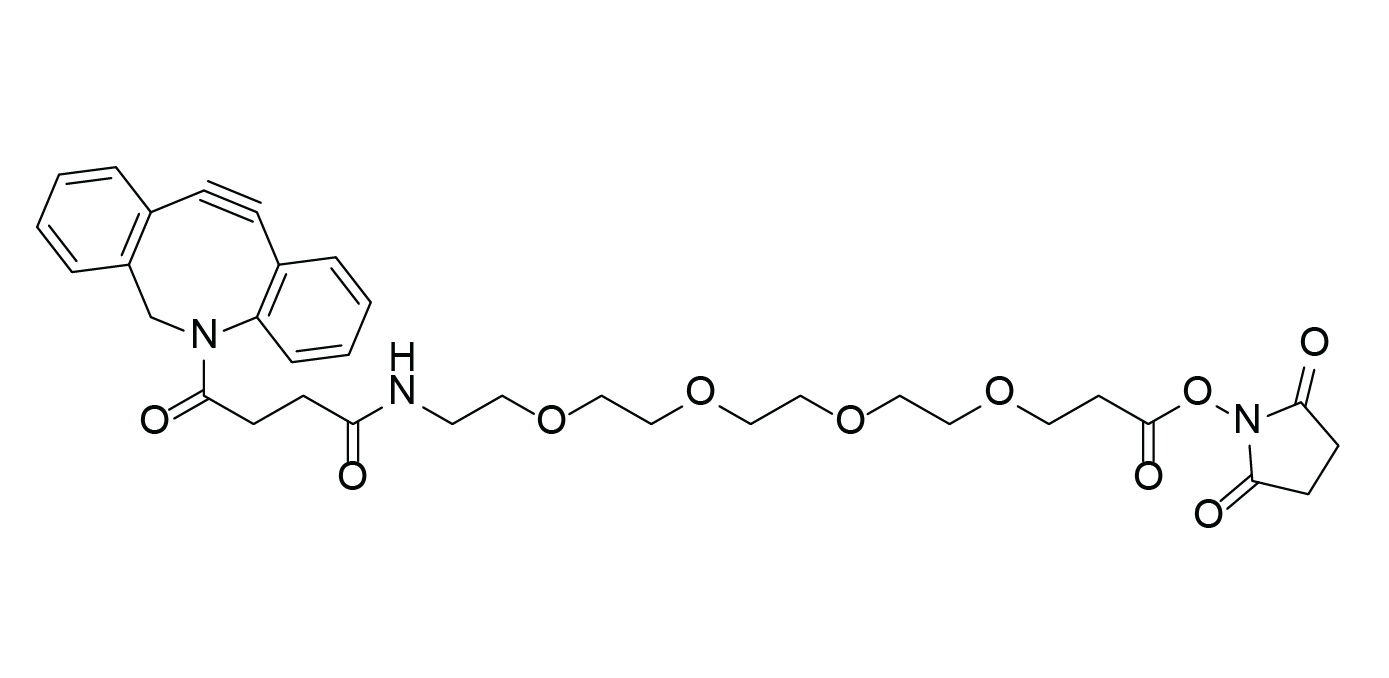
DBCO-PEG4-NHS ester
Bifunctional linker
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCL-038-5 | € 90,00 |
| 10 mg | BCL-038-10 | € 140,00 |
| 50 mg | BCL-038-50 | € 480,00 |
Chemical Properties
-
Molecular Formula
C34H39N3O10
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark, dry, inert gas
-
Molecular Weight
649.7 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
light yellow gel
-
CAS Number
1427004-19-0
-
Additional name
DBCO-PEG4-succinimidyl Ester, 2,5-Dioxo-1-pyrrolidinyl 20-(11,12-didehydrodibenz[b,f]azocin-5(6H)-yl)-17,20-dioxo-4,7,10,13-tetraoxa-16-azaeicosanoate
Dibenzocyclooctyne-PEG4-succinimidyl Ester -
Solubility
DMSO, DCM, DMF
-
Preparation/Handling
For a 10 mM solution add 154 μL per 1 mg.
Product Information
Heterobifunctional Linker for Amine Coupling and Copper‑Free Click Chemistry
DBCO‑PEG4‑NHS ester is a heterobifunctional crosslinker combining:
• NHS ester for selective coupling to primary amines (proteins, peptides, antibodies, amine‑modified oligonucleotides)
• DBCO for copper‑free SPAAC (strain‑promoted azide–alkyne cycloaddition) with azide partners
This dual reactivity enables orthogonal bioconjugation under mild, biocompatible conditions—ideal for crosslinking, surface functionalization, and controlled modifications where a hydrophilic PEG4 spacer improves solubility, reduces aggregation, and minimizes steric hindrance.
Why DBCO‑PEG4‑NHS Ester?
Traditional conjugation can suffer from poor selectivity or harsh conditions that jeopardize sensitive biomolecules. DBCO‑PEG4‑NHS ester provides a precise, stable and efficient platform for molecular engineering in pharmaceutical development, diagnostics, and advanced biotechnology.
Key Advantages
- Dual Reactivity & PEG4 Spacer
Introduces a DBCO moiety onto primary amines, unlocking copper‑free click chemistry. The PEG4 backbone increases polarity and provides a defined spacer to reduce steric clashes and aggregation—particularly useful for complex biomolecules.
Example: Post‑synthetic DBCO labeling of amino‑modified oligonucleotides, where DBCO is not compatible with standard solid‑phase synthesis conditions. - Fast & Efficient SPAAC
Once installed, the DBCO group reacts rapidly with azides to form stable triazoles under catalyst‑free conditions—delivering high to near‑quantitative conversions with simplified purification. - Biocompatible, Cleaner Workflows
Copper‑free conditions and mild buffers help preserve biomolecule structure and function, supporting sensitive proteins and nucleic acids. - Versatile Handling
PEG4 improves aqueous compatibility and allows use in mixed aqueous/organic systems with common water‑miscible co‑solvents when needed, supporting both bench bioconjugation and materials/surface applications.
Applications in Research and Industry
- Antibody–Drug Conjugates (ADCs): Install DBCO on lysine residues for downstream SPAAC to azide cargos
- Surface & Hydrogel Modifications: Controlled immobilization on functionalized materials
- Antibody, Aptamer & Oligo Functionalization: Post‑synthetic DBCO introduction for modular click labeling
Comparison of baseclick DBCO Linkers
| Feature / Property | DBCO‑PEG4‑DBCO | DBCO‑PEG4‑NHS Ester | DBCO‑C6‑NHS Ester |
| Type | Homo‑bifunctional linker | Heterobifunctional linker | Heterobifunctional linker |
| Functional Groups | 2 × DBCO | DBCO + NHS ester | DBCO + NHS ester |
| Spacer | PEG4 (hydrophilic) | PEG4 (hydrophilic) | C6 (hydrophobic) |
| Primary Use | Crosslinking, nucleic acid circularization | Introduce DBCO to amine‑containing biomolecules | Introduce DBCO to amine‑containing biomolecules |
| Solubility | Aqueous compatible | Aqueous compatible | Organic solvents (DCM, THF, EtOAc) |
| Click Reaction | SPAAC (copper‑free) | SPAAC (copper‑free) | SPAAC (copper‑free) |
| Ideal For | Dual azide coupling, precise crosslinking | Proteins, peptides, oligos (amine modification) | Organic synthesis, hydrophobic conjugates |
| Key Advantage | Enables two‑step conjugation for defined products | Adds DBCO under mild conditions; PEG4 improves polarity | Suitable for nonpolar environments and surface functionalization |
LITERATURE
Equipping natural killer cells with cetuximab through metabolic glycoengineering and bioorthogonal reaction for targeted treatment of KRAS mutant colorectal cancer, X. Wang et al., 2021, ACS Chemical Biology, Vol. 16(4), p. 724-730.
https://doi.org/10.1021/acschembio.1c00022
Enzyme‐Based Synthetic Protein Nanoparticles as Colloidal Antioxidants, A. Mauser et al., 2023, Advanced Therapeutics, 2300007.
https://doi.org/10.1002/adtp.202300007
Construction of artificial cilia from microtubules and kinesins through a well-designed bottom-up approach, R. Sasaki et al., 2018, Nanoscale, Vol. 10(14), p. 6323-6332.
https://doi.org/10.1039/C7NR05099B
Novel approaches to label the surface of S. aureus with DBCO for click chemistry-mediated deposition of sensitive cargo, T. H. Baryakova et al., 2025, Bioconjugate Chemistry, Vol. 36(6), 1157-1168.
https://pubs.acs.org/doi/10.1021/acs.bioconjchem.4c00575
FAQ
-
Do I need copper or special catalysts?
No. After NHS‑amine coupling installs the DBCO, subsequent SPAAC with azides is copper‑free, enabling biocompatible workflows.
-
What buffers/conditions are suitable for the NHS coupling step?
Use mild, slightly basic, amine‑coupling buffers commonly employed for NHS chemistry (e.g., PBS‑based or carbonate systems). Prepare fresh solutions and avoid prolonged exposure to moisture to minimize NHS hydrolysis. Always validate on your specific biomolecule.
-
Is DBCO PEG4 NHS ester compatible with aqueous systems?
Yes. The PEG4 spacer increases polarity, supporting aqueous or mixed aqueous/organic conditions. For poorly soluble substrates, introduce a water‑miscible co‑solvent (e.g., DMSO or DMF) while maintaining biomolecule stability.
-
How does this compare to DBCO C6 NHS ester?
DBCO‑PEG4‑NHS offers higher polarity and reduced steric hindrance versus the more hydrophobic C6 variant—making it preferable when aqueous handling and biomolecule compatibility are priorities. Choose DBCO‑C6‑NHS for non‑polar or surface‑focused workflows where hydrophobicity is beneficial.
-
Is DBCO PEG4 NHS ester cost effective?
Yes. You benefit from efficient, copper‑free click reactions, simplified purification, and less reagent waste. In addition, baseclick offers competitive pricing, making DBCO‑PEG4‑NHS ester a budget‑friendly option without compromising quality.
-
What if my biomolecule is sensitive to organic co solvents?
Start with aqueous buffers enabled by PEG4 polarity and minimize co‑solvent content. If required, optimize stepwise (buffer composition, concentration, temperature) to preserve activity; validate on small scale before scale‑up.

