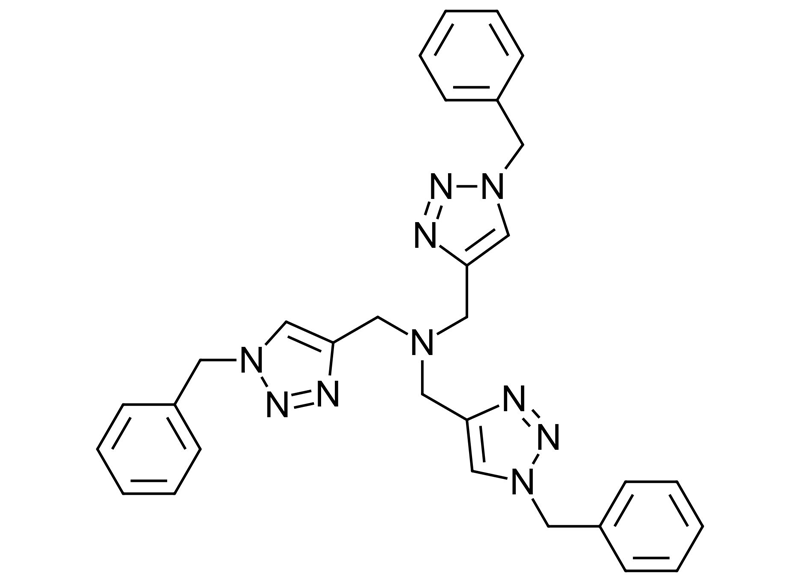
TBTA | Tris((3-benzyl-1H-1,2,3-triazol-4-yl)methyl)amine
"baseclick-grade" ligand in high quality for optimal yield in organic solution
| Size | Catalog No. | Price |
|---|---|---|
| 10 mg | BCMI-002-10 | € 40,00 |
| 100 mg | BCMI-002-100 | € 130,00 |
Chemical Properties
-
Molecular Formula
C30H30N10
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C
-
Molecular Weight
530.63 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
white to off white powder
-
CAS Number
510758-28-8
-
Additional name
Tris((3-benzyl-1H-1,2,3-triazol-4-yl)methyl)amine
Product Information
TBTA | Tris((3-benzyl-1H-1,2,3-triazol-4-yl)methyl)amine
TBTA (Tris((3-benzyl-1H-1,2,3-triazol-4-yl)methyl)amine) is a tertiary amine that comprises three 1,2,3 triazole groups. The TBTA ligand is a polytriazolylamine ligand, which is frequently used in coordination chemistry, namely in copper(I) catalyzed azide-alkyne cycloaddition (CuAAC). The copper ion Cu(I) will be stabilized in its +1 oxidation state by the TBTA ligand. Hereby, TBTA function is to protect it from oxidation and disproportionation. During this, TBTA can also increase the catalytic activity of the copper to increase the reaction rate of CuAAC. Due to TBTA’s stability under standard reaction conditions, TBTA ligand can be utilized as a versatile choice for other catalysis processes such as in polymerization reactions or for the synthesis of functional nanomaterials.
Understanding TBTA
Chemical structure & properties of TBTA
TBTA ligand is a symmetrical molecule consisting of a central tertiary amine connected to three identical substituents. All substituents are connected by a methylene group to the C4 of a 1,2,3-triazole. All triazoles have a benzyl group linked to the N1 of the triazole. These structures make TBTA an ideal ligand for chelating copper ions. TBTA forms a trigonal bipyramidal structure with Cu(II) species which can be easily reduced to a Cu(I) complex by reducing agents such as sodium ascorbate. The structure of the Cu(I) TBTA ligand complex forms an unusual dinuclear dication, where one triazole unit is bridging two Cu(I) ions. The copper (I) complex is stabilized from disproportion or oxidation by oxygen from TBTA to increase its lifetime to enable it to be a stable tolerant catalytic species for CuAAC reaction. The chelating of copper by TBTA ligand also reduces the likelihood of copper ions to catalyze the formation of reactive oxygen species which could lead to product degeneration. The benzyl groups of TBTA function as an unpolar group to increase the solubility of the TBTA ligand in organic solvents to enable Click Chemistry for non water-soluble starting materials. baseclick is offering TBTA and other materials necessary to set up your own CuAAC reactions in high quality.
Functionality of TBTA in click chemistry
The specific form of the Copper TBTA ligand complex forms a bulky structure where the triazole rings of TBTA function as a protective layer around the metal center of the complex shielding it from oxidating agents or other copper ions necessary for disproportionation.
Advantages of using TBTA
Enhanced reaction conditions
TBTA ligand and other polytriazolylamine ligands function as a protective layer for the central copper ion when forming the complex around it with their triazole rings. These layers of ligands such as TBTA protect the center copper ion from oxidation or other unwanted side reactions to destroy the catalyst. These polytriazolylamine ligands can be optimized on the chemical structure linked to the 1N group of the triazole leading to different solubility of the constructed ligands while maintaining the same potential to protect the Cu(I) oxidation state as TBTA ligand.
Therefore, TBTA containing CuAAC reactions do not need to be performed under strict oxygen free conditions which includes the degassing of solvents. Thus, TBTA and its analogs enable much easier reaction setup for CuAAC.
Increased reaction rates & yields
Studies comparing CuAAC reactions kinetics for reactions with and without a ligand as TBTA found that the usage of TBTA ligand can increase reactions speed up to about 100 times. In combination with the potential of TBTA to prevent the formation of reactive oxygen species by copper-ions the usage of TBTA enables to perform CuAAC reactions in short time with high yields without degradation by the formation of reactive species. The increase of reaction kinetics allows for use of lower reaction temperatures which minimize the formation of side products while maintaining reasonable reaction times.
Versatility & compatibility
The molecule structure of TBTA ligand containing functional groups such as 1,2,3-triazoles, benzyl-groups and a tertiary amine enables TBTA to be non-reactive to most functional groups used in organic- or bio-chemistry. Therefore, TBTA is well suited to be used as ligand for CuAAC reactions for a wide variety of substrates including biomolecules as sensitive RNA or Proteins.
Applications of TBTA in research & industry
Bioconjugation
TBTA ligand or similar ligands are an essential part of every CuAAC reaction performed on biomolecules such as nucleic acids or antibodies. The TBTA function to protect the integrity of biomolecules during CuAAC can be used to label biomolecules, for example with fluorescence tags, to analyze them using Imaging methods such as fluorescent microscopy. Another TBTA function is to use CuAAC to covalently connect biomolecules to targeting agents for drug delivery or use TBTA ligand in a reaction to connect antibody drug conjugates (ADCs) covalently.
Material science
TBTA or a similar ligand is always necessary when the biorthogonal copper catalyzed alkyne azide cycloaddition (CuAAC) should be performed in high efficiency. TBTA protects the Cu(I) needed for reaction catalysis from oxidation, disproportionation or blockage by external nucleophiles. Therefore, the TBTA ligand supported CuAAC to be performed with almost no side reaction and with catalyst species that have longer lifetimes than non-ligand supported CuAAC. These TBTA ligand supported reactions enable the production of new materials in high yields with specific control over their structure to form the properties desired by their developer.
Pharmaceutical development
The copper catalyzed alkyne azide cycloaddition (CuAAC) is one of the most important reactions for the synthesis of new drug candidates or in updated synthesis processes due to its high yields, its easy setup and work up of the reactions, its stable reaction product of selective 1,4 substituted triazoles and its high tolerance towards other functional groups. To match all these necessary criteria for drug development CuAAC reactions need a stabilization of the copper catalyst. The usage of copper stabilizing ligands such as TBTA achieves this necessary stabilization making CuAAC an ideal reaction in drug discovery and in the development of new synthesis processes.
Future trends in TBTA research
Emerging areas of research
TBTA ligand supported click chemistry is already used in drug development such as in the synthesis of ADC or PROTACs. But the great properties of click chemistry, combined with ligands such as TBTA, offer enormous potential in the automated synthesis of small molecule drug discovery. The idea is to produce libraries of molecules with either an azide or an alkyne and combine them through TBTA supported Click Chemistry automatically to produce enormous numbers of new small molecules. The triazole formed by Click Chemistry with TBTA is also an interesting medicinal motif on its own. This reinforces the use of CuAAC for this purpose. TBTA mediated CuAAC is for its stable covalent linkage between building blocks also of great interest in disciplines such as material science, biomolecular imaging or chemical biology.
Innovative TBTA ligand modifications
There are various attempts ongoing to modify TBTA ligand to optimize its properties to specific requests. Starting from simply modification of the chemical structure of the groups attached to the triazoles to modify the polarity of the ligand, which led to the development of ligands such as THPTA, BTTAA or BTTES. These similar ligands to TBTA have similar impact to CuAAC in terms of reaction kinetics and protection of the Cu(I) oxidation state but can have quite different properties in terms of solubility in certain solvent systems. There are also much further advanced chemical changes to TBTA ligand as the development of a covalent cage connecting the triazoles to form a molecular sphere around the central copper to shield it from any interfering nucleophile as glutathione. Another approach is to connect a TBTA precursor covalently to DNA, which could be delivered to cells together with DNA and connected copper, to enable CuAAC reactions in living cells.
LITERATURE
Copper-Catalyzed Reaction Cascade: Direct Conversion of Alkynes into N-Sulfonylazetidin-2-imines, M. Whiting et al., 2006, Angew. Chem. Int. Ed., Vol. 45, p. 3157-3161.
https://doi.org/10.1002/anie.200503936
Copper-Catalyzed Multicomponent Reactions: Securing a Catalytic Route to Ketenimine Intermediates and their Reactivities, E. J. Yoo et al., 2009, Curr. Org. Chem., Vol. 13, p. 1766-1776.

