
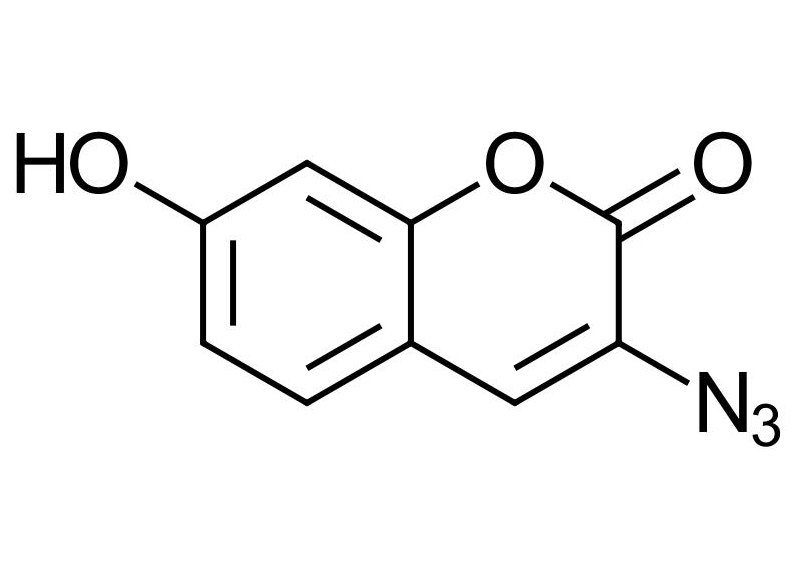
3-Azido-7-hydroxycoumarin
Switch-on dye to label DNA/RNA
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCFA-047-5 | € 135,00 |
| 10 mg | BCFA-047-10 | € 200,00 |
Chemical Properties
-
Molecular Formula
C9H5N3O3
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark
-
Molecular Weight
203.15 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
amber to brown crystalline solid
-
CAS Number
817638-68-9
-
Additional name
3-Azido-7-hydroxy-chromen-2-on; 3-Azido-7-
hydroxy-chroman-2-one -
Excitation (max)
before click reaction: 260 nm
after click reaction: 404 nm -
Emission (max)
before click reaction: 391 nm
after click reaction: 480 nm -
Solubility
DMSO, DMF, MeOH, MeCN
-
Preparation/Handling
For a 10 mM solution add 492 μL to 1 mg.
Product Information
A Click-Activated, Fluorogenic Dye for Bioorthogonal Labeling and Live-Cell Imaging
3-Azido-7-hydroxycoumarin is a highly responsive, fluorogenic coumarin-based dye designed for bioorthogonal labeling through copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC). Unlike traditional dyes that emit background fluorescence regardless of conjugation, this compound remains non-fluorescent until it undergoes a click reaction with an alkyne-functionalized molecule.
The fluorescence is “switched on” by triazole formation, which extends the dye’s π-conjugation system resulting in bright, stable emission ideal for precise imaging applications.
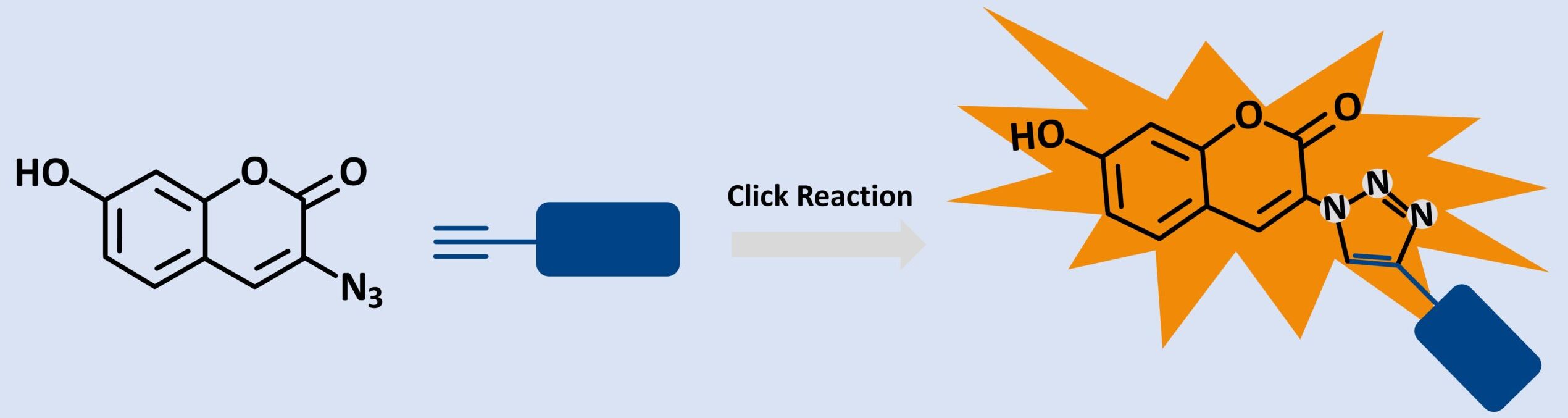
Key Features and Photophysical Properties
- Fluorogenic design – minimal background signal
- Azide group at position 3 – enables CuAAC click reaction
- Hydroxy group at position 7 – supports photophysical performance
- Excitation λmax: 404 nm
- Emission λmax: 477 nm
- Water-soluble, photostable, and cell-permeable
- Compatible with live-cell imaging and pH-stable environments.
Click-Activated Coumarin Dye for High-Contrast Live-Cell Imaging
3-Azido-7-hydroxycoumarin offers a suite of features that make it exceptionally well-suited for precision bioimaging and molecular labeling:
- Click-activated fluorescence minimizes background signal, enhancing imaging clarity
- Bioorthogonal azide functionality enables highly selective and non-invasive bioconjugation
- Coumarin-based core provides superior photostability and a high quantum yield for long-term tracking
- Excellent cell and tissue permeability allows effective use in live-cell and in vivo environments
- Highly compatible with fluorescence microscopy, particularly for DNA labeling in proliferating cells using 5-Ethynyl-2’-deoxyuridine (EdU)
3-Azido-7-hydroxycoumarin is a highly responsive substance with a wide range of applications in various fields:
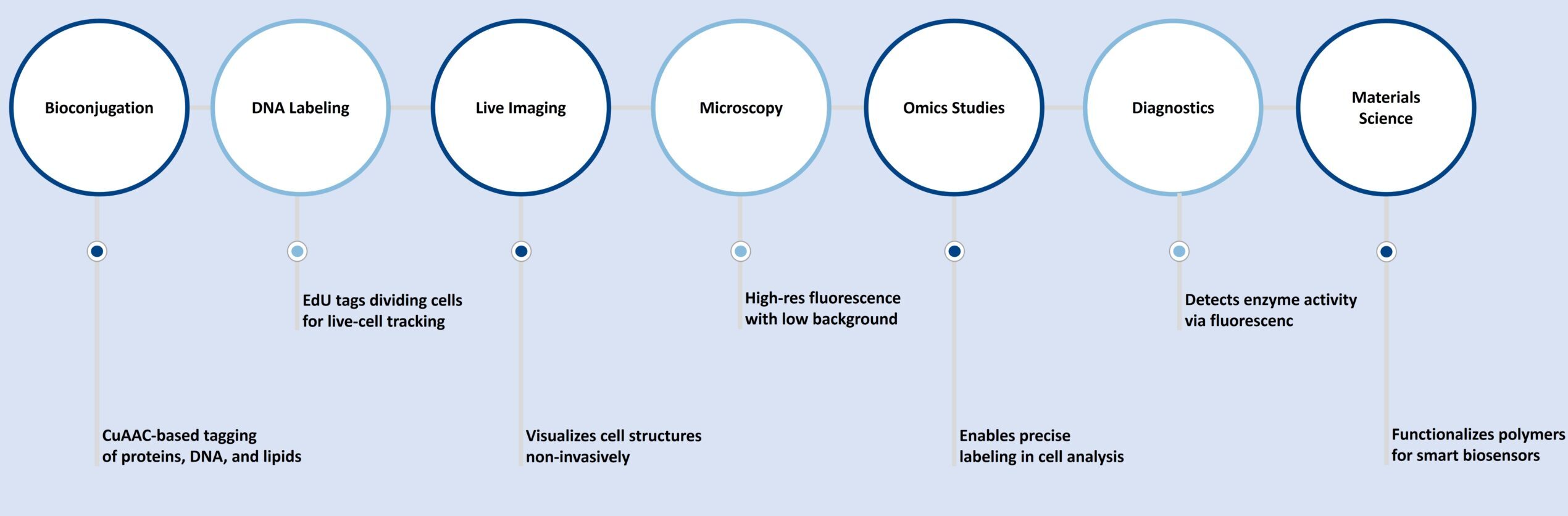
Applications
- Live-cell imaging with low background
- DNA labeling via EdU incorporation and click detection
- Protein or glycan visualization in fixed or live cells
- Development of biosensors and optically controlled systems
- Targeted delivery and diagnostic assays in complex biological samples
3-Azido-7-hydroxycoumarin redefines precision fluorescence labeling by combining:
- The compact, water-soluble coumarin scaffold
- The selective activation power of click chemistry
- And robust, high signal photophysical performance
This dye is a smart choice for researchers who require clean, no-wash, high-sensitivity detection in real-time biological systems.
Whether it’s tracking proliferating cells, visualizing protein interactions, or engineering smart biosensors, this dye has carved out a vital niche in modern bioimaging, diagnostics, and molecular engineering.
Literature
Imaging the glycome, Laughlin et al, 2009, Sci USA, Vol. 106, p. (1):12
https://doi.org/10.1073/pnas.0811481106
Fluorogenic “click” reaction for labeling and detection of DNA in proliferating cells, Li et al, 2010, Biotechniques, Vol. 49, p. (1):525
https://doi.org/10.2144/000113463
A Fluorogenic 1,3-Dipolar Cycloaddition Reaction of 3-Azidocoumarins and Acetylenes, Sivakumar et al, 2004, Org. Lett., Vol. 6, p. (24):4603
https://doi.org/10.1021/ol047955x
Site-Directed Immobilization of Bone Morphogenetic Protein 2 to Solid Surfaces by Click Chemistry, C. Siverino et al., 2018, Journal of Visualized Experiments, Vol. 133, e56616.
Characterization and Scaled-Up Production of Azido-Functionalized Silk Fiber Produced by Transgenic Silkworms with an Expanded Genetic Code, H. Teramoto et al., 2019, International Journal of Molecular Sciences, Vol. 20(3), p. 616.
https://doi.org/10.3390/ijms20030616
A lipid transfer protein knockout library reveals ORP9-ORP11 dimer mediating PS/PI(4)P exchange at the ER-trans Golgi contact site to promote sphingomyelin synthesis, B. Cabukusta et al., 2023, bioRxiv.
https://doi.org/10.1101/2023.06.02.543249
Trifunctional lipid probes for comprehensive studies of single lipid species in living cells, D. Höglinger, 2017, Proceedings of the National Academy of Sciences, Vol. 114(7), p. 1566-1571.

