
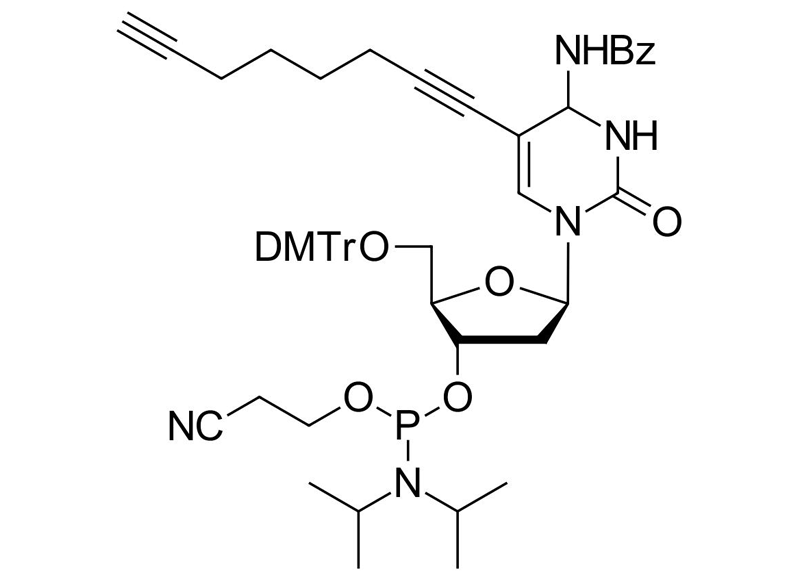
C8-Alkyne-dC-CE Phosphoramidite
Clickable phosphoramidite for Oligo synthesis
| Size | Catalog No. | Price |
|---|---|---|
| 250 mg | BCA-07-250 | € 260,00 |
Chemical Properties
-
Molecular Formula
C54H60N5O8P
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C dry, inert gas
-
Molecular Weight
938.06 g/mol
-
Purity
≥ 95% (31P NMR)
-
Physical State
white to off-white solid
-
CAS Number
1021300-97-9
-
Additional name
C8-Alkyne-dC-phosphoramidite, 4-N-Benzoyl-5-(octa-1,7-diynyl)-3´-O-[(2-cyanoethoxy)(diisopropylamino)-phosphono)]-5´-O-(4,4´-dimethoxytrityl)-2’-deoxycytidine
-
Preparation/Handling
This phosphoramidite is used under standard conditions for solid phase synthesis of oligonucleotides.
Product Information
Advanced Tool for Oligonucleotide Labeling via Click Chemistry
C8-Alkyne-dC-CE Phosphoramidite is a high-performance modified nucleoside tailored for solid-phase oligonucleotide synthesis. Featuring a flexible C8 spacer, it enhances accessibility and conjugation efficiency in Copper(I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) reactions. This reagent enables site-specific labeling, bioorthogonal conjugation, and multiplexed imaging, while preserving DNA integrity and hybridization properties.
Key Features & Benefits
C8 Spacer for Enhanced Flexibility: Improves accessibility for conjugation, boosting click reaction yields and enabling efficient post-synthesis modifications.
Alkyne Functionality for CuAAC: Supports precise bioorthogonal labeling using copper-catalyzed click chemistry, ideal for sensitive and targeted applications.
High Coupling Efficiency: Ensures reliable incorporation during oligonucleotide synthesis, maintaining structural integrity and functional performance.
Preserved Hybridization: Unlike earlier alkynyl modifications, C8-Alkyne-dC maintains duplex stability, enabling accurate probe design and hybridization-based assays.
Expanded Labeling Capabilities: Supports single, dual, or triple modifications—ideal for complex probe architectures in diagnostics, therapeutics, and imaging.
Why Choose C8-Alkyne-dC-CE Phosphoramidite?
Before its introduction, oligonucleotide labeling faced several limitations:
- Rigid Structures: Short alkynyl linkers restricted conjugation efficiency.
- Hybridization Disruption: Some modifications interfered with duplex formation.
- Limited Multiplexing: Traditional methods constrained multi-labeling strategies.
C8-Alkyne-dC-CE Phosphoramidite overcomes these challenges by offering:
- Superior Flexibility for efficient conjugation.
- Stable Hybridization for reliable probe performance.
- Versatile Labeling Options for advanced oligo designs.
Applications
- PCR probe development
- Targeted drug delivery systems
- Fluorescent and biotin labeling
- Molecular diagnostics and biosensors
- Nucleic acid-based therapeutics
LITERATURE
Postsynthetic DNA Modification through the Copper-Catalyzed Azide–Alkyne Cycloaddition Reaction, P. M. E. Gramlich et al., 2008, Angew. Chemie Int. Ed., Vol. 47, p. 8350–8358.
https://doi.org/10.1002/anie.200802077
Click–Click–Click: Single to Triple Modification of DNA, P. M. E. Gramlich et al., 2008, Angew. Chemie Int. Ed., Vol. 47, p. 3442-3444.
https://doi.org/10.1002/anie.200705664
Generation and Characterization of a DNA-GCN4 Oligonucleotide-Peptide Conjugate: The Impact DNA/Protein Interactions on the Sensitization of DNA, P. Wityk et al., 2020, Molecules, Vol. 25(16), p. 3630.
https://doi.org/10.3390/molecules25163630
FAQ
-
What is C8-Alkyne-dC-CE Phosphoramidite used for?
It is a modified nucleoside used in solid-phase oligonucleotide synthesis for site-specific labeling and bioorthogonal conjugation via Copper(I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC).
-
What makes the C8 spacer important?
The C8 spacer provides enhanced flexibility, improving accessibility for conjugation and increasing the efficiency of click chemistry reactions.
-
Does this modification affect DNA hybridization?
No. C8-Alkyne-dC maintains duplex stability, ensuring reliable hybridization and functional performance in downstream applications.
-
Can I use this phosphoramidite for multiplexed labeling?
Yes. It supports single, dual, or triple modifications, making it ideal for multiplexed probe designs in diagnostics and imaging.
-
Is it compatible with standard oligo synthesis protocols?
Absolutely. It offers high coupling efficiency and integrates seamlessly into standard phosphoramidite synthesis workflows.
-
What are typical applications for this reagent?
It’s used in:
- PCR probe development
- Targeted drug delivery systems
- Fluorescent labeling
- Molecular diagnostics
- Nucleic acid therapeutics
-
How does it compare to traditional alkynyl-modified nucleotides?
Traditional alkynyl nucleotides often had short, rigid linkers that limited conjugation and disrupted hybridization. C8-Alkyne-dC overcomes these issues with flexibility and stability.
-
Is this product suitable for sensitive biological systems?
Yes. Its bioorthogonal alkyne group and low copper requirement make it suitable for copper-sensitive environments.
-
What kind of conjugates can be attached using CuAAC?
You can attach a wide range of azide-functionalized molecules, including fluorophores, biotin, drugs, and affinity tags.
Where can I purchase C8-Alkyne-dC-CE Phosphoramidite?
It is available through baseclick’s webshop and authorized distributors. For bulk orders or technical support, contact baseclick directly.

