
Ferrocene Azide
Organometallic tag for labeling DNA/RNA
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCFA-019-5 | € 170,00 |
| 10 mg | BCFA-019-10 | € 300,00 |
Chemical Properties
-
Molecular Formula
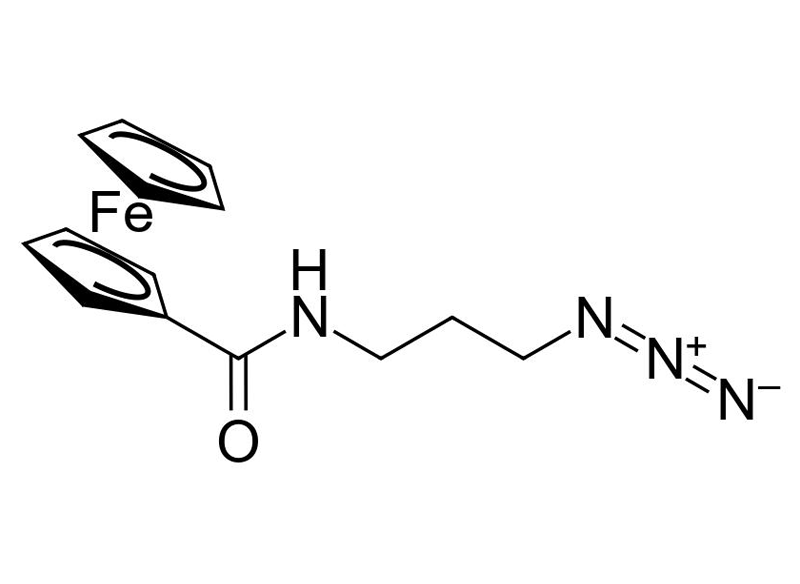
C14H16FeN4O
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dry, inert gas
-
Molecular Weight
312.15 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
yellow to light orange solid
-
CAS Number
1887761-56-9
-
Solubility
DMSO
-
Preparation/Handling
For a 10 mM solution add 320 μL to 1 mg.
Product Information
Redox-Active Click Chemistry Building Block
Ferrocene Azide is a versatile organometallic reagent combining chemical stability, bioorthogonal reactivity, and electrochemical signaling. Through CuAAC click chemistry, it enables precise functionalization for advanced applications in biosensing, diagnostics, materials science, and medicinal chemistry.

Why Ferrocene Azide Matters
Before its introduction, researchers faced:
Limited ferrocene functionalization: No universal reactive handle for molecular modifications.
Poor bioconjugation efficiency: Difficult attachment of biomolecules or drugs.
Weak electrochemical markers: Existing probes lacked stability and sensitivity.
Unclear biological roles: Hindering rational design of ferrocene-based therapeutics.
Ferrocene Azide solves these challenges by offering:
Bioorthogonal click reactivity: Azide group enables safe, selective CuAAC under mild conditions.
Dual functionality: Ferrocene provides redox activity; azide acts as a modular linkage point.
Device integration: Compatible with nanomaterials (e.g., PEDOT-N₃, graphene nanoribbons) for biosensor fabrication.
Enhanced probe stability: Ferrocene-tagged biomolecules outperform fluorescent labels in robustness and sensitivity.
Facilitates SAR studies: Supports structural tuning and electrochemical profiling for drug development.
Facilitates SAR and mechanistic studies: Enables structural tuning and electrochemical profiling for drug development.
Applications of Ferrocene Azide
| Domain | Examples of Application |
| Medicinal Chemistry | Anticancer, antibacterial, antiprotozoal agents via ferrocene-triazole hybrids |
| Electrochemical Sensing | DNA/RNA detection using EDDA and HPLC-ECD systems |
| Bioelectronics | Thrombin detection using functionalized OECTs |
| Nanomaterial Interfaces | Graphene nanoribbons with aptamer click-conjugation for IL6 sensing |
| Enzyme Inhibition | Targeting aromatase, HDAC, carbonic anhydrase, and HIV-1 entry |
| Material Science | Surface functionalization and smart coatings through click chemistry |
Key Features
- Organometallic stability for robust conjugates
- Redox-active marker for electrochemical readouts
- Bioorthogonal compatibility for complex biological systems
- Modular design for multifunctional interfaces
Why Choose Ferrocene Azide?
It is more than a reactive intermediate, it is a gateway to multifunctional bioorganic interfaces, enabling breakthroughs in:
- Diagnostics
- Therapeutics
- Molecular electronics
Ferrocene-triazole conjugates continue to deliver potent biological activity while driving innovation in biosensing, drug design, and smart materials.
LITERATURE
“Clickable” Organic Electrochemical Transistors. W. Knoll et al., 2022, J. Am.Chem. Soc. Au 2, 2778−2790.
Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects. A. Saeed et al., 2023, Molecules, 28, 5765.
Nanocomposites of Graphene with Ferrocene or Hemin: Preparation and Application in Electrochemical Sensing. B. Zhou et al., 2025, J. Nanomater. 2015, 1-9.
Electrochemically active DNA probes: Detection of target DNA sequences at femtomole level by high-performance liquid chromatography with electrochemical detection, S. Takenaka et al. 1994, Anal. Biochem., 218: 436-443.
https://doi.org/10.1006/abio.1994.1203
Ferrocene-oligonucleotide conjugates for electrochemical probing of DNA T. Ihara et al., 1996, Nucleic Acids Res., 24: 4273-4280.
https://doi.org/10.1093/nar/24.21.4273
DNA-arrays with electrical detection: A label-free low cost technology for routine use in life sciences and diagnostics. P. Liepold et al., 2005, Bioelectrochem., 67: 143-150.
https://doi.org/10.1016/j.bioelechem.2004.08.004
Electrically detected displacement assay (EDDA): a practical approach to nucleic acid testing in clinical or medical diagnosis, P. Liepold et al., 2008, Anal. Bioanal. Chem., 391: 1759-1772.
https://doi.org/10.1007/s00216-008-2045-5
Interface Engineering of “Clickable” Organic Electrochemical Transistors toward Biosensing Devices Devices, G. E. Fenoy et al., 2023, ACS Appl. Mater. Interfaces, Vol. 15(8), p. 10885–10896.
https://doi.org/10.1021/acsami.2c21493
“Clickable” graphene nanoribbons for biosensor interfaces, R. Hasler et al., 2024, Nanoscale Horiz., 9, 598-608.
https://doi.org/10.1039/D3NH00590A
FAQ
-
What is Ferrocene Azide used for?
Ferrocene Azide is a versatile building block for CuAAC click chemistry, enabling functionalization of biomolecules, nanomaterials, and surfaces for applications in biosensing, drug design, and molecular electronics.
-
Why is Ferrocene Azide better than traditional ferrocene derivatives?
It introduces an azide group, allowing bioorthogonal click reactions under mild conditions. This solves previous limitations in ferrocene functionalization and improves biocompatibility and integration with biomolecules.
-
Is Ferrocene Azide safe for biological systems?
Yes, when used under optimized CuAAC conditions. The azide group enables selective conjugation without interfering with native biomolecules, and reactions can be performed under mild, aqueous conditions.
-
What makes Ferrocene Azide useful in sensing applications?
Ferrocene provides a stable redox signal, making it an excellent electrochemical marker for DNA/RNA detection, enzyme assays, and biosensor platforms such as organic electrochemical transistors (OECTs).
-
Can Ferrocene Azide be used in live-cell systems?
Direct live-cell labeling is limited due to copper requirements in CuAAC. However, picolyl-azide strategies or optimized copper-chelating ligands can reduce toxicity for biological environments.
-
Which biomolecules can be labeled?
Proteins, peptides, nucleic acids, and small molecules containing alkyne groups. It also integrates with nanomaterials like graphene and PEDOT for advanced device fabrication.
-
How does Ferrocene Azide improve probe stability?
Ferrocene-tagged biomolecules are more robust and reusable than fluorescent labels, offering long-term electrochemical stability and high sensitivity.
-
What storage conditions are recommended?
Store at −20 °C, protected from light and moisture. Avoid repeated freeze-thaw cycles. The product is slightly sensitive to oxygen, therefore flood the sample with protective gas before long term storage.
-
Does Ferrocene Azide have biological activity?
Yes. Ferrocene-triazole conjugates have shown anticancer, antimicrobial, and enzyme inhibition properties, making them valuable in medicinal chemistry research.
-
Are there complementary products?
Yes. baseclick offers a portfolio of alkyne-functionalized biomolecules and click chemistry reagents for conjugation workflows.

