
THPTA | Tris(3-hydroxypropyltriazolylmethyl)amine
"baseclick-grade" water-soluble ligand in high quality for optimal yield
| Size | Catalog No. | Price |
|---|---|---|
| 10 mg | BCMI-006-10 | € 33,00 |
| 50 mg | BCMI-006-50 | € 70,00 |
| 100 mg | BCMI-006-100 | € 105,00 |
Chemical Properties
-
Molecular Formula
C18H30N10O3
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C
-
Molecular Weight
434.5 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
white colored powder
-
CAS Number
760952-88-3
-
Additional name
Tris(3-hydroxypropyltriazolylmethyl)amine
-
Solubility
Water or DMSO/t-BuOH (Click Solution)
Product Information
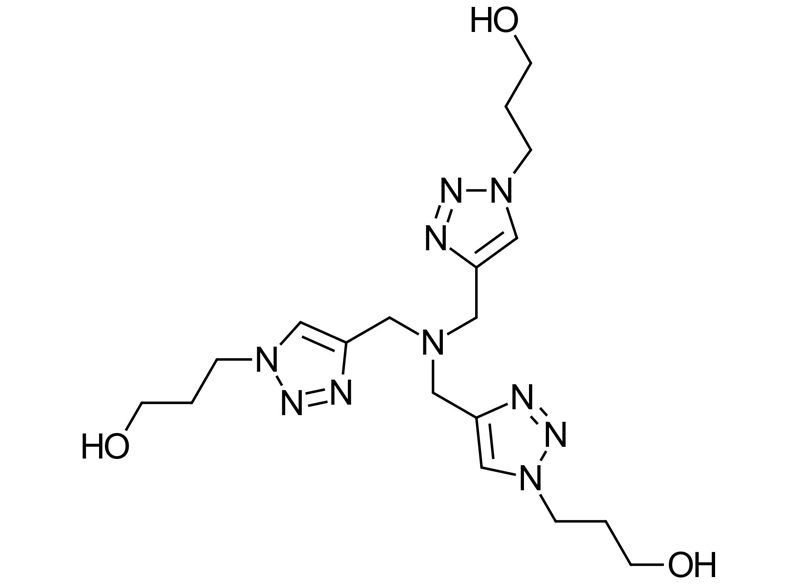
What is THPTA?
THPTA ligand is a symmetrical molecule consisting of a central tertiary amine connected to three identical substituents. Each substituent is linked by a methylene group to the C4 position of a 1,2,3-triazole, and each triazole has a hydroxypropyl group attached to the N1 position. These structures make THTPA an ideal ligand for chelating copper ions in the biorthogonal CuAAC reaction.
In CuAAC, THPTA forms a cage-like complex with its 1,2,3-tetrazoles around the copper ion. This complexation stabilizes the copper ion in its +1 oxidation state, protecting it from disproportionation and oxidation by oxygen. This stabilization increases the lifetime of the copper ion, enabling a stable and tolerant catalytic species for the CuAAC reaction. Furthermore, the THPTA ligand’s ability to chelate copper reduces the catalyzing effect of copper ions on the formation of reactive oxygen species, which could otherwise lead to product degradation. The hydroxypropyl groups of THPTA serve as a polar group, increasing the solubility of the THPTA in pure aqueous environments.
Application:
THPTA ligand enables CuAAC reactions to be carried in pure aqueous systems due to its excellent water solubility. This eliminates the need for organic solvent mixtures, which can otherwise compromise the structural integrity of sensitive biomolecules by denaturing their secondary or tertiary structures.
Advantages:
THPTA offers several advantages in nucleic acid modification, protein coupling/ bioconjugation, creation of fluorescent probes for cellular imaging, drug discovery and pharmaceutical research, particularly in the context with the copper catalyzed azide-alkyne cycloaddition (CuAAC) reactions:
- Lower Copper Catalyst Load: THPTA enables CuAAC reactions to be performed with lower loads of the copper catalyst. This is due to the elongation of the lifetime of the active Cu(I) catalyst, which remains stable for a longer period of time in the THPTA complex.
- Reduced Cytotoxic Effects: The reduced copper concentration when using the THPTA ligand minimizes the cytotoxic effects of copper. This is particularly important in biological applications where cell viability is crucial.
- Shielding from Side Reactions: THPTA effectively shields copper from catalyzing side reactions, further reducing cytotoxicity and preventing the formation of reactive oxygen species (ROS) that can damage cellular components.
- Shorter Reaction Time: THPTA reduces the reaction time for CuAAC, thereby decreasing the duration of copper exposure. This is beneficial for maintaining the integrity of cell structures during fluorescent labeling.
- Efficient Fluorescent Labeling: These combined effects enable efficient fluorescent labeling of cell structures while causing minimal damage to the cell structure. This is particularly advantageous in microscopy, where preserving the native state of the sample is critical for accurate imaging.
Next to THPTA use as a ligand in CuAAC which all its benefits in improving labeling in biochemistry, production and modification of medicinal nanostructures or covalent linking of targeting structures to drugs as in ADC or siRNA delivery approaches, the THPTA copper complex can be used for selective cleavage of thiazolidines in proteins. The THPTA mediated release of a -oxo-aldehydes can be subsequently used for further protein modification on specific structures, which is a crucial step in the development of new protein or enzyme therapeutics.
The unique properties and advantages of THPTA have already enabled baseclick and ClickSeq technologies to develop a next-generation sequencing kit based on THPTA-supported CuAAC (copper(I) catalyzed azide-alkyne cycloaddition) reactions for primer extension. These kits, such as RNAseq Library Prep kit, DNAseq Library prep kit, and Poly (A)-ClickSeq kits, offer several benefits for sequencing applications. They enable efficient and precise primer extension, ensuring high-quality sequencing results with minimal degradation of the target molecules.
LITERATURE
Copper-Catalyzed Azide–Alkyne Click Chemistry for Bioconjugation, S. Presolski et al., 2011, Current Protocols of Chemical Biology, Vol. 3(4), p. 153-162.
https://doi.org/10.1002/9780470559277.ch110148
Selection and Characterization of SARS-CoV-2 Spike Binding Clickmers, Günter Mayer et al., 2026, Chembiochem. 2026 Jan;27(1):e202500733

