Proliferations Assays: Modern Methods for Cell Growth Analysis
What are Cell Proliferation Assays?
Cell proliferation assays are carried out to determine the viability of the cells. They provide important insights in fields such as cancer research, drug development, toxicology, and regenerative medicine. These assays detect changes in cell number, metabolic activity, or DNA synthesis to assess cell viability. Kit cell proliferation assays fall into two primary methods. On the one hand, indirect methods (e.g., metabolic assays, ATP concentration measurement) which offer a quick assessment but do not distinguish between dividing and non-dividing cells. On the other hand, direct methods (e.g. DNA synthesis cell proliferation assays like EdU or BrdU incorporation) provide precise measurements of proliferating cells.
How Do Cell Proliferation Assays Work?
So far, the most common Kit cell proliferation assays which use direct methods, are based on the detection of the newly synthesized DNA, by incorporation of a thymidine analogue such as 5’-bromo-2’-deoxyuridine (BrdU), H3-thymidine or 5-ethynyl-2′-deoxyuridine (EdU) during cell division. In these cell proliferation assays the detection of these nucleobases are different. In case of cell proliferation assays based on BrdU it is obtained by antibody conjugation. Detection of proliferations assay based on H3-thymidine is done by its intrinsic radioactive activity. And finally EdU Kit cell proliferation assays using Click Chemistry conjugation to link a fluorophore to EdU which is incorporated in new synthesized DNA. Are you more interested in how EdU is incorporated and how you get the fluorophore into the cells?
General protocol of cell proliferation assays. A Step-by-Step Process:
- Cells are seeded in appropriate culture conditions.
- The proliferation marker (e.g. EdU) is added to the medium.
- After incubation, cells are fixed and permeabilized.
This allows labeling using a detection method (e.g. click chemistry with a fluorophore for EdU cell proliferation assays).
- Analysis with fluorescence microscopy, flow cytometry (FACS), or High-throughput screening (HTS) to quantify labeled cell.
But how do I know which proliferations assay is right for me? Here are some key factors that affect measurement accuracy:
Kit cell proliferation assay selection: Choosing between direct and indirect methods based on sensitivity needs. Indirect methods can give you a fast and cheep hint on the proliferation, but real and reliable readouts can only be guaranteed by using direct methods.
Cell type and growth rate: Different cells proliferate at different rates.
Labeling efficiency and detection sensitivity: Ensuring complete incorporation of proliferation markers. The EdU cell proliferation assays can detect proliferating cells more effectively and are therefore also more sensitive than the BrdU cell proliferation assays.
Types of Cell Proliferation Assays
There are a variety of cell proliferation assay methods based on various cell functions such as enzyme activity, cell membrane permeability, cell adherence, ATP production, co-enzyme production, and nucleotide uptake activity. Such as metabolic kit cell proliferation assays (e.g. MTT, XTT, WST-1) which assess mitochondrial activity but do not differentiate between live and dividing cells. DNA synthesis cell proliferation assays (e.g. EdU, BrdU, 3H-thymidine incorporation) directly label newly synthesized DNA. Direct cell counting using imaging techniques or automated cytometry. Multiple cell proliferation assays exist, and the choice of method depends on the laboratory resources available, the types of cells/tissues to be studied, and the specific experimental goals.
Advanced Analysis Methods of Proliferations Assay
Kit Cell Proliferation Assays Cytometry
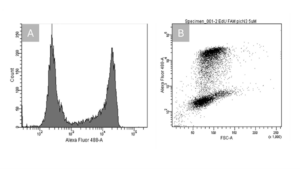
Flow cytometry is a widely used technique for analysing the physical and chemical properties of cells or particles as they pass through a laser. It is commonly used to assess cell populations based on parameters such as size, complexity and fluorescence intensity. In the case of cell proliferation analysis, the latter is used. To explain this in more detail, an example of FACS measurement has been performed using the EdU Kit Cell Proliferation Assays cytometry. In the figure below HeLa cells have been incubated with the nucleoside analog EdU (5-ethynyl-2’-deoxyuridine) for 2 hours, which incorporates into newly synthesized DNA during the S-phase of the cell cycle. A click chemistry reaction is then performed, labeling the EdU with the fluorescent dye 6-FAM, an analog of Alexa Fluor 488, allowing for detection via flow cytometry.
The first peak (located between 102 and 103), using EdU Kit Cell Proliferation Assays cytometry, represents cells that did not incorporate EdU, indicating these cells were not synthesizing new DNA and therefore have not been in the S-phase of the cell cycle (non-proliferating cells). These cells have very low fluorescence because they did not incoperate the 6-FAM label.
The second peak, which is shifted towards the right (between 104 and 105), corresponds to cells that have actively synthesized DNA and thus incorporated EdU. The shift in fluorescence intensity occurs because the newly synthesized DNA is now labeled with 6-FAM, which emits a fluorescent signal detectable by flow cytometry. The greater the fluorescence intensity, the more EdU was incorporated, indicating higher levels of DNA synthesis and thus active cell proliferation.
Learn more about flow cytometry here: Flow Cytometry Glossary
Kit Cell Proliferation Assays Imaging
Fluorescence imaging has become a fundamental tool for biomedical applications. Its intravital imaging capacity lays in the conventional wavelength range (400–950 nm). There is a wealth of new fluorescent reporter technologies for tagging of many cellular and subcellular processes in vivo. Such as cell proliferation assay imaging methods that offer new ways to visualize and quantify fluorescent markers distributed in tissues. There are five key methods for measuring cell proliferation—indirect measures (including MTT, MTS, WST-1, and XTT), cell cycle markers (Ki67, cell nuclear antigen (PNCA) and phospho-histone H3), dye dilution assays (carboxyfluorescein succinimidyl ester (CFSE)), DNA synthesis methods (BrdU and EdU Kit cell proliferation assays imaging), and live-cell imaging (fluorescent proteins live-cell imaging) is a transformative technique in cell biology that allows the dynamics of living cells to be observed in real time. Key components of a cell imaging system include the incubation chamber, fluorescence illumination system, automated stage, digital image acquisition and analysis software, and environmental systems to control conditions. Image analysis software quantifies fluorescence intensity to determine proliferation rates.
Learn more about Live Cell Imaging Glossary here: Live Cell Imaging Glossary
Kit Cell Proliferation Assays High Throughput Screening
Laboratory robotics are widely used for toxicity testing, which is a major cause of failure in drug discovery and development. While robust toxicological testing is carried out, efficiency could be improved by identifying compounds with cytotoxic properties during large-scale cell analysis and screening. This approach has advantages in terms of time and cost efficiency. The use of automated high-throughput screening (HTS) imagers in combination with cell proliferation assays is becoming more widespread. To explain this in more detail, an example of HTS measurement has been performed using the EdU Kit Cell Proliferation Assays High troughput Screening. The figure below shows an HTS analysis (fluorescence plate reader) of HeLa cells after EdU (5-ethynyl-2’-deoxyuridine) feeding after 2 hours. The click reaction was performed using a 6-FAM dye. Comparison study of different EdU cell proliferation assays when varying the number of cells analyzed. Using the EdU Sensitive Kit, it is possible to reduce background and to enhance the sensitivity of the cell proliferation assay. This improvement in signal intensity and signal-to-background ratio allows scientists to continue to enable the analysis of lower cell numbers, as demonstrated by the EdU Kit Cell Proliferation Assays High troughput Screening (see Figure below). In fact, where the standard test failed, the new version (BCK-EdUPro-HTS488) was able to reliably detect cell contents down to 500 cells per well (in a 96-well plate setup).
For a high-sensitivity EdU-based cell proliferation assays, check out: EdU Cell Proliferation Assay for High-Throughput Screening
Proliferations Assays Applications
Kit Cell Proliferation Assays in Research
Drug toxicity is one of the key areas of interest in pharmacology, where around a third of drug candidates are rejected. It is concerned with the concept of cell viability, which describes the number and proportion of living and dead cells in a population. Cell proliferation assays are ideal for screening potential anti-cancer compounds based on their effect on cell proliferation. Cell proliferation assays are reliable, accessible and scalable viability assays based on cell death. Fluorescence imaging and biomarkers are essential for efficient drug toxicity screening. This is important in cancer research to understand tumour growth and therapy resistance. Cell proliferation assays can monitor differentiation and self-renewal capabilities, providing insight into cancer treatment and cancer stem cell research. The advent of fluorescent labelling of cells has enabled precise measurements of proliferation, lifespan and hierarchical relationships in normal and neoplastic tissues.