
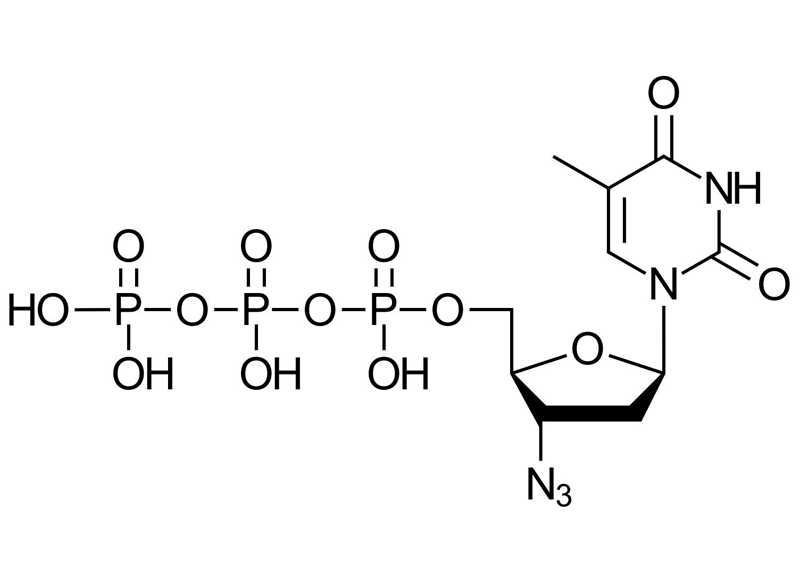
3′-Azido-2′,3′-ddTTP
Triphosphate for modifying of 3'END RNA or ssDNA
| Size | Catalog No. | Price |
|---|---|---|
| 1 µmol | BCT-28-S | € 250,00 |
| 5 µmol | BCT-28-L | € 850,00 |
Chemical Properties
-
Molecular Formula
C10H16N5O13P3
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C
-
Molecular Weight
507.18 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
100 mM clear aquaeous solution; colorless
-
CAS Number
149022-21-9 (sodium salt)
92586-35-1 (free acid)
-
Absorption (max)
λmax = 267 nm
-
Ɛ (max)
10,900 cm-1M-1
-
Additional name
3′-Azido-ddTTP, AZTTP, AZT-TP, Zidovudine
Triphosphate, Azidothymidine Triphosphate
Product Information
A Nucleotide Analogue for the Selective 3´End Labeling of Nucleic Acids by Click Chemistry (CuAAC/SPAAC) or Staudinger Ligation
3′-Azido-2′,3′-ddTTP is a chemically modified analogue of Uridine triphosphate (UTP) featuring an azido (N₃) group replacing the 3′-OH group of the sugar moiety. This modification enables efficient labeling through click chemistry reactions:
- CuAAC (Copper-catalyzed alkyne–azide cycloaddition) – Produces a single regioisomer and is easily scalable for product development.
- SPAAC (Strain-promoted alkyne–azide cycloaddition) – Catalyst-free, simplifying purification, but yields two regioisomers.
The absence of the 3′-OH group makes 3′-Azido-2′,3′-ddTTP a chain-terminating nucleotide in polymerization reactions such as IVT. Unlike other modified DNA or RNA nucleotides (e.g., EdUTP or EUTP), it cannot be incorporated internally during nucleic acid synthesis. Instead, it is ideal for post-synthetic, 3′-end-specific introduction of a single azido-modified nucleobase using enzymes such as T7 polymerase or terminal deoxynucleotidyl transferase (TdT).
This nucleotide can replace T in DNA or U in RNA for 3′-end labeling. Detailed protocols for conjugation with fluorescent dyes, biotins, linkers, and cell-targeting ligands via click chemistry are available from baseclick.
Role in NGS Sequencing
The chain-terminating property of 3′-Azido-2′,3′-ddTTP is utilized in baseclick’s ClickSeq NGS kits for generating cDNA libraries, improving sequencing workflows by reducing artifacts and enhancing reproducibility.
Azide Functionality and Bioorthogonal Ligation
The azido group (R–N₃) is bioorthogonal, meaning it does not react with natural biomolecules. Under physiological conditions, azides selectively react with:
- Alkynes → Form triazoles via Cu(I)-catalyzed or strain-promoted cycloaddition (e.g., DBCO, BCN).
- Phosphines → Form amides via Staudinger ligation.
These reactions enable precise chemical modifications without interfering with biological systems.
Application areas
- 3′-End labeling of nucleic acids (ssDNA, mRNA) for imaging, affinity tagging, or functionalization via click chemistry.
- Sequencing workflows:
- Acts as a chain-terminating nucleotide during reverse transcription or cDNA synthesis.
- Enables primer conjugation without enzymatic ligation, improving efficiency and reducing artifacts in RNA-seq workflows.
Used in baseclick and ClickSeq kits to overcome common RNA-seq bottlenecks such as chimeras, recombination, fragmentation bias, and ligation inefficiencies, while preserving library complexity.
LITERATURE
Site-specific terminal and internal labeling of RNA by poly(A) polymerase tailing and copper-catalyzed or copper-free strain-promoted click chemistry, M. L. Winz et al., 2012, Nucleic Acids Res., Vol. 40, p. 1–13.
https://doi.org/10.1093/nar/gks062
Chemoenzymatic Preparation of Functional Click-Labeled Messenger RNA, S. Croce et al., 2020, ChemBioChem, Vol. 21, p. 1641-1646.
https://doi.org/10.1002/cbic.201900718
Application and design considerations for 3′-end sequencing using click-chemistry, M. K. Jensen et al., 2021, Methods in Enzymology, Vol. 655, p. 1-23.
https://doi.org/10.1016/bs.mie.2021.03.012
FAQ
-
What is 3′-Azido-2′,3′-ddTTP?
It is a chemically modified thymidine triphosphate analogue with an azido (N₃) group replacing the 3′-OH group, enabling selective 3′-end labeling of nucleic acids via click chemistry.
-
How does it differ from other modified nucleotides?
Unlike EUTP or EdUTP, 3′-Azido-2′,3′-ddTTP cannot be incorporated internally during nucleic acid synthesis. It acts as a chain terminator, making it ideal for post-synthetic 3′-end labeling.
-
Which enzymes can incorporate 3′-Azido-2′,3′-ddTTP?
T7 RNA polymerase
Terminal deoxynucleotidyl transferase (TdT)
-
What click chemistry reactions are compatible?
CuAAC (Copper-catalyzed alkyne–azide cycloaddition) – Single isomer, scalable.
SPAAC (Strain-promoted alkyne–azide cycloaddition) – Catalyst-free, easy purification.
Staudinger ligation – Reaction with phosphines forming amides.
-
What are the main applications?
3′-end labeling of ssDNA and mRNA for imaging or functionalization.
Chain termination in sequencing workflows (e.g., ClickSeq kits).
Primer conjugation without enzymatic ligation.
-
Can it replace thymidine or uridine?
Yes. It can substitute T in DNA or U in RNA for 3′-end labeling.
-
Is it used in sequencing kits?
Yes. It is a key component in baseclick’s ClickSeq NGS kits, improving RNA-seq workflows by reducing artifacts and enhancing reproducibility.
-
Does it affect DNA polymerases?
No. It acts as a chain terminator but does not interfere with DNA polymerase activity.

