
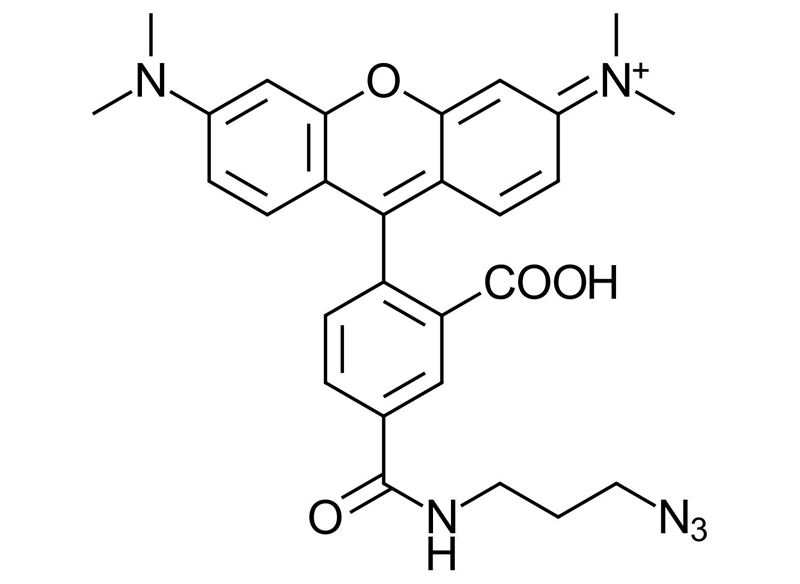
5-Carboxytetramethylrhodamine Azide (5-TAMRA-Azide)
TAMRA fluorescent dye to label DNA/RNA
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCFA-008-5 | € 100,00 |
| 10 mg | BCFA-008-10 | € 150,00 |
Chemical Properties
-
Molecular Formula
C28H28N6O4
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark
-
Molecular Weight
512.56 g/mol
-
Purity
≥ 95% (LCMS)
-
Physical State
pink to dark red colored solid
-
CAS Number
1192590-89-8
-
Additional name
5-TAMRA-Azide; 5-Carboxytetramethylrhodamine Azide, 5-((3-Azidopropyl)carbamoyl)-2-(6-(dimethylamino)-3-(dimethyliminio)-3H-xanthen-9-yl)benzoate
-
Excitation (max)
DMSO or DMF: 546 nm
-
Emission (max)
DMSO or DMF: 579 nm
-
Ɛ (max)
DMSO or DMF = 91,000 cm-1M-1
-
Solubility
DMSO, DMF, MeOH
-
Preparation/Handling
For a 10 mM solution add 195 μL to 1 mg.
Product Information
The red fluorophore for precision click chemistry and live‑cell lmaging
5‑Carboxytetramethylrhodamine Azide (5‑TAMRA‑Azide) is a red‑fluorescent dye widely used for biomolecule labeling through click chemistry. With excitation and emission maxima near λex ≈ 555 nm and λem ≈ 580 nm, it delivers bright, photostable fluorescence compatible with Alexa Fluor® 555, DyLight 549, and TRITC filter sets. Its robust performance under physiological conditions makes it ideal for imaging, tracking, and advanced bioconjugation workflows.
Key features that make 5‑TAMRA‑Azide in bioorthogonal labeling
- High fluorescence intensity: Strong, reliable red signal for imaging and detection.
- Fast, efficient click reactions: CuAAC labeling completes in under an hour with high yield.
- Bio‑orthogonal specificity: Selective azide–alkyne ligation ensures minimal background.
- Stability and durability: Triazole linkage remains intact under diverse conditions.
- Biologically inert: No interference with native cellular processes.
- Live‑cell compatibility: Enables real‑time DNA synthesis detection and intracellular drug tracking.
- Multiplexing capability: Works with other fluorophores for multi‑parameter analysis; can act as a quencher for 6‑FAM in dual‑labeled probes.
Applications in Research and Industry
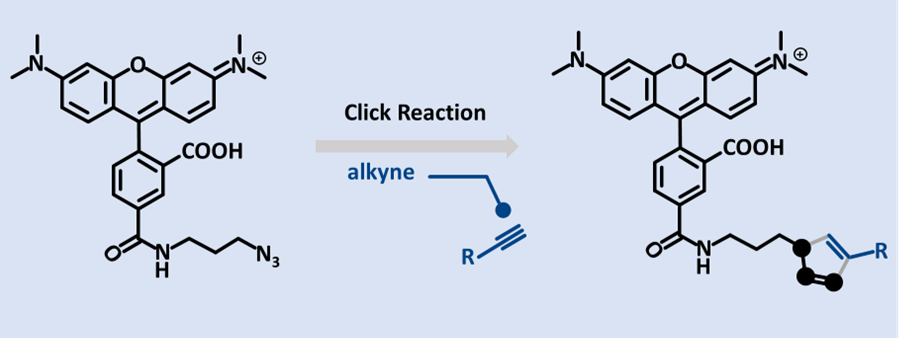
1. Click Chemistry & Bioorthogonal Reactions
- Copper‑catalyzed azide–alkyne cycloaddition (CuAAC) for stable, bio‑inert conjugates.
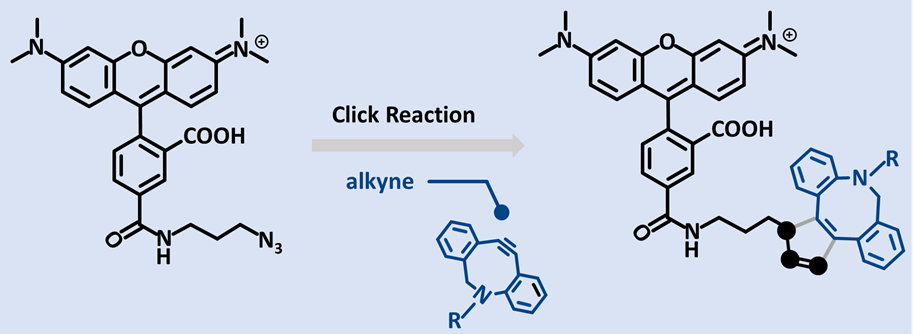
- Copper‑free SPAAC with DBCO or BCN derivatives for sensitive biological systems.
2. FRET & Reaction Kinetics Studies
5‑TAMRA‑Azide serves as a fluorescence acceptor in Fluorescence Resonance Energy Transfer (FRET) assays, enhancing sensitivity in DNA sequencing, molecular interaction studies, and protein synthesis analysis. It can also be employed in reaction kinetics studies.
3. Live-Cell DNA Imaging & Drug Tracking
5‑TAMRA‑Azide is cell-permeable, allowing DNA synthesis detection in live cells without fixation or permeabilization. It is used for EdU (5-ethynyl-2′-deoxyuridine) labeling, ensuring that only newly synthesized DNA detected, providing specific insights into DNA replication dynamics. Additionally, it is employed in click chemistry-based fluorescent labeling to visualize and track intracellular distribution and behavior of drugs in living systems.
4. Applications in DNA & Protein Labeling
Widely used in post synthetic DNA labeling, 5‑TAMRA‑Azide facilitates efficient incorporation of multiple labels via CuAAC click chemistry, applicable in molecular diagnostics and nanotechnology. It has demonstrated strong fluorescent signals, lead to be applied to peptides, proteins, polysaccharides, viruses, and cells, extending its utility across various biological research areas.
With its versatile labeling capabilities, high selectivity, and robust fluorescence properties, 5‑TAMRA‑Azide remains a powerful tool for biomolecule detection, protein synthesis tracking, drug localization studies, and biomedical research.
Enhanced Variant: 5‑TAMRA‑PEG3‑Azide
Moreover, baseclick offers 5-TAMRA-PEG3-Azide, an enhanced fluorescence labeling solution with a PEG3 (polyethylene glycol) spacer for improved solubility, reduced steric hindrance, and better biomolecule accessibility. Ideal for click chemistry, FRET assays, DNA labeling, and protein tagging, it ensures high fluorescence stability and minimal background interference, making it perfect for precise bioconjugation in complex environments.
Literature
Postsynthetic DNA Modification through the Copper-Catalyzed Azide–Alkyne Cycloaddition Reaction, P. M. E. Gramlich et al., 2008, Angew. Chemie Int. Ed., Vol. 47, p. 8350–8358.
https://doi.org/10.1002/anie.200802077
Click–Click–Click: Single to Triple Modification of DNA, P. M. E. Gramlich et al., 2008, Angew. Chemie Int. Ed., Vol. 47, p. 3442-3444.
https://doi.org/10.1002/anie.200705664
A chemical method for fast and sensitive detection of DNA synthesis in vivo, A. Salic et al., 2008, Proc. Natl. Acad. Sci. U. S. A., Vol. 105, p. 2415–2420.
https://doi.org/10.1073/pnas.0712168105
Fluorescent labelling of in situ hybridisation probes through the copper-catalysed azide-alkyne cycloaddition reaction, S. Hesse et al., 2016, Chromosome Research, Vol. 24, p. 299–307.
https://doi.org/10.1007/s10577-016-9522-z
Genetic Encoding of a Bicyclo[6.1.0]nonyne-Charged Amino Acid Enables Fast Cellular Protein Imaging by Metal-Free Ligation, A. Borrmann et al., 2012, ChemBioChem, Vol. 13(14), p. 2094-2099.
https://doi.org/10.1002/cbic.201200407
New insights into the intracellular distribution pattern of cationic amphiphilic drugs, M. Vater et al., 2017, Scientific Reports, Vol 7, p. 44277.
https://doi.org/10.1038/srep44277
The Cyanobacterial “Nutraceutical” Phycocyanobilin Inhibits Cysteine Protease Legumain, I. V. L. Wilkinson et al., 2022, ChemBioChem, Vol. 24(5), p. e202200455.

