
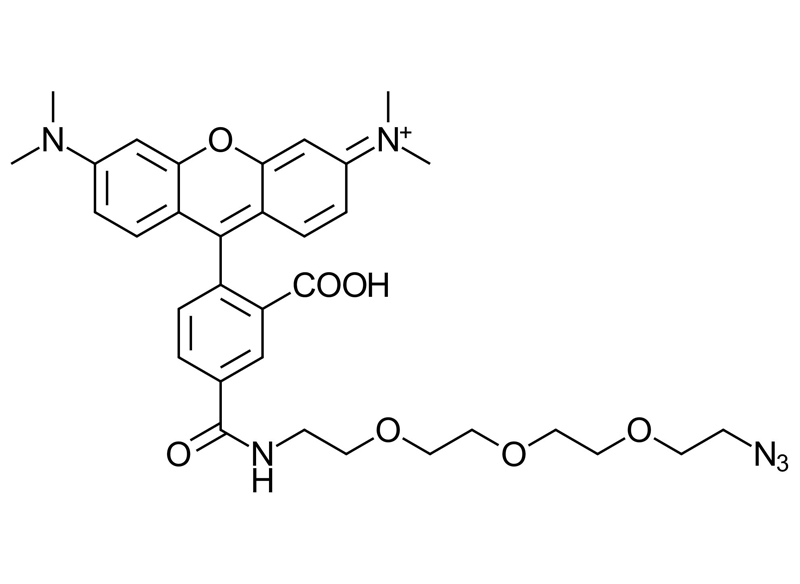
5-Carboxytetramethylrhodamine-PEG3-Azide (5-TAMRA-PEG3-Azide)
Fluorescent dye to label DNA/RNA
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCFA-037-5 | € 80,00 |
| 10 mg | BCFA-037-10 | € 125,00 |
| 100 mg | BCFA-037-100 | € 500,00 |
Chemical Properties
-
Molecular Formula
C33H38N6O7
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark
-
Molecular Weight
630.69 g/mol
-
Purity
≥ 90% (HPLC)
-
Physical State
pink to dark red solid
-
CAS Number
1380486-02-1
-
Additional name
5-Carboxytetramethylrhodamine-PEG3-Azide
-
Excitation (max)
DMF or DMSO: 546 nm
-
Emission (max)
DMF or DMSO: 579 nm
-
Ɛ (max)
91,000 cm-1M-1
-
Solubility
DMSO, DMF, MeOH, slightly soluble in water
-
Preparation/Handling
For a 10 mM solution add 158 μL to 1 mg.
Product Information
An orange-red fluorophore for precision for all click chemistry applications
5-Carboxytetramethylrhodamine-PEG3-Azide (5-TAMRA-PEG3-Azide) is an orange red fluorescent dye widely used for the labeling of biomolecules using click chemistry. With excitation and emission maxima at approximately λ_ex ≈ 555 nm and λ_em ≈ 580 nm, it provides bright, photostable fluorescence compatible with Alexa Fluor® 555, DyLight® 549, and TRITC filter sets. Its reliable performance under physiological conditions makes it well suited for imaging, molecular tracking, and advanced bioconjugation workflows.
5-TAMRA-PEG3-Azide is a derivative of 5-TAMRA-Azide that incorporates a hydrophilic PEG3 spacer between the azide functional group and the fluorophore core. This spacer reduces steric hindrance during conjugation while significantly increasing water solubility. The PEG3 linker is particularly advantageous for labeling bulky biomolecules or sensitive biological systems, as it minimizes nonspecific interactions and reduces the need for organic solubilizing additives such as DMSO.
Key features that make 5‑TAMRA‑PEG3-Azide in bioorthogonal labeling
- High fluorescence intensity: Strong, reliable orange-red signal for imaging and detection.
- Fast, efficient click reactions: CuAAC labeling completes in under an hour with high yield.
- Bio‑orthogonal reaction: Selective azide–alkyne conjugation ensures maximal selectivity.
- Stability and durability: Triazole linkage remains intact under almost all conditions.
- Biologically inert: No interference with native cellular processes.
- Reduced steric interference: The PEG3 spacer minimizes steric interactions between the dye and the target molecule.
- Multiplexing capability: Works with other fluorophores for multi‑parameter analysis; can act as a quencher for FAM in dual‑labeled probes. For molecular beacons usually 5-TAMRA-Azide is used because there is no need for the additional spacer.
Applications in Research and Industry
Click Chemistry & Bioorthogonal Reactions:
Copper‑catalyzed azide–alkyne cycloaddition (CuAAC): enables the formation of stable, bio inert conjugates with reduced steric hindrance.
Copper‑free SPAAC labeling:
Compatible with DBCO and other strained alkyne derivatives, allowing labeling of sensitive biological systems.
FRET & Reaction Kinetics Studies:
TAMRA dyes can serve as fluorescence acceptors in Fluorescence Resonance Energy Transfer (FRET) assays, enhancing sensitivity in DNA sequencing, molecular interaction studies, and protein synthesis analysis. 5-TAMRA-PEG3-Azide can also be applied in reaction kinetics studies.
DNA & Protein Labeling:
Widely used in post synthetic DNA labeling, 5‑TAMRA-PEG3‑Azide facilitates efficient incorporation of multiple labels via CuAAC click chemistry, applicable in molecular diagnostics and nanotechnology. It has demonstrated strong fluorescent signals, can be applied to peptides, proteins, polysaccharides, viruses, and cells, extending its utility across various biological research areas. For example 5-Carboxytetramethylrhodamine-PEG3-Azide is used as detection reagent in all 555 variants of baseclick´s EdU cell proliferation detection kits.
With its versatile labeling capabilities, high selectivity, and robust fluorescence properties, 5‑TAMRA‑PEG3-Azide remains in combination with its mother dye 5-TAMRA-Azide a powerful tool for biomolecule detection, protein synthesis tracking, drug localization studies, and biomedical research.
LITERATURE
Postsynthetic DNA Modification through the Copper-Catalyzed Azide–Alkyne Cycloaddition Reaction, P. M. E. Gramlich et al., 2008, Angew. Chemie Int. Ed., Vol. 47, p. 8350–8358.
https://doi.org/10.1002/anie.200802077
Click–Click–Click: Single to Triple Modification of DNA, P. M. E. Gramlich et al., 2008, Angew. Chemie Int. Ed., Vol. 47, p. 3442-3444.
https://doi.org/10.1002/anie.200705664
A chemical method for fast and sensitive detection of DNA synthesis in vivo, A. Salic et al., 2008, Proc. Natl. Acad. Sci. U. S. A., Vol. 105, p. 2415–2420.
https://doi.org/10.1073/pnas.0712168105
Chemoenzymatic Preparation of Functional Click-Labeled Messenger RNA, S. Croce et al., 2020, ChemBioChem, Vol. 21(11), p. 1641-1646.
https://doi.org/10.1002/cbic.201900718
Synthesis and biological evaluation of anthracene-9,10 dione derivatives as CK2 inhibitors, P. López-Rojas et al., 2023, Results in Chemistry, Vol. 6, p. 100997.
https://doi.org/10.1016/j.rechem.2023.100997
FAQ
-
What is 5‑TAMRA‑PEG3‑Azide used for?
5‑TAMRA‑PEG3‑Azide is used for fluorescent labeling of biomolecules via click chemistry. It provides bright, photostable orange‑red fluorescence for applications such as imaging, molecular tracking, FRET assays, DNA/protein labeling, and reaction kinetics analysis.
-
What are the excitation and emission maxima?
The dye exhibits:
- Excitation maximum: ~555 nm
- Emission maximum: ~580 nm
This makes it fully compatible with Alexa Fluor® 555, DyLight® 549, and TRITC filter sets.
-
What advantages does the PEG3 spacer provide?
The PEG3, a hydrophilic linker:
- Reduces steric hindrance during conjugation
- Improves water solubility
- Minimizes nonspecific interactions
- Reduces the need for organic solvents such as DMSO
This makes it especially beneficial for labeling bulky biomolecules, sensitive proteins, and living cells.
-
Is 5‑TAMRA‑PEG3‑Azide suitable for CuAAC click chemistry?
Yes. It is optimized for copper‑catalyzed azide–alkyne cycloaddition (CuAAC) and typically reacts within <1 hour with high yield, forming stable triazole linkages.
-
Can it be used for copper‑free SPAAC reactions?
Yes. Although designed primarily for CuAAC, the azide can also react with strained alkynes such as DBCO, enabling copper‑free labeling of more sensitive biological samples.
-
Is this fluorophore bioorthogonal?
Yes. The azide functional group reacts selectively with alkynes and does not interfere with native cellular functions, making the reagent suitable for biological imaging and live‑cell workflows.
-
Can 5‑TAMRA‑PEG3‑Azide be used for multiplex fluorescence experiments?
Absolutely. Its spectral characteristics make it compatible with multiparameter imaging workflows.
It can also act as a quencher for FAM in dual‑labeled probes.
For molecular beacons without spacing requirements, 5‑TAMRA‑Azide (without PEG3) may be preferable.
-
What biomolecules can be labeled using 5‑TAMRA‑PEG3‑Azide?
Applicable targets include:
- DNA and RNA (post‑synthetic labeling)
- Peptides and proteins
- Polysaccharides
- Viruses and viral particles
- Whole cells
The reagent is also used as a detection dye in all 555‑labelled baseclick EdU cell proliferation kits.
-
How stable is the fluorescent linkage?
The resulting triazole bond is extremely robust and remains stable under physiological, chemical, and thermal conditions, providing durable fluorescent labeling.
-
How should the product be stored?
Store at –20 °C, protected from light and moisture. Avoid repeated freeze–thaw cycles to maintain dye integrity.
-
Is the dye compatible with aqueous labeling reactions?
Yes. Thanks to the PEG3 spacer, the reagent is highly water‑soluble, enabling efficient labeling in aqueous or minimally organic environments.
-
Does baseclick offer related fluorophores?
Yes. baseclick provides a wide range of azide‑ and alkyne‑functional fluorophores including:
5‑TAMRA‑Azide (without PEG3)
- FAM‑Azide
- Cy5‑Azide
- as well as DBCO‑fluorophores for SPAAC labeling.

