
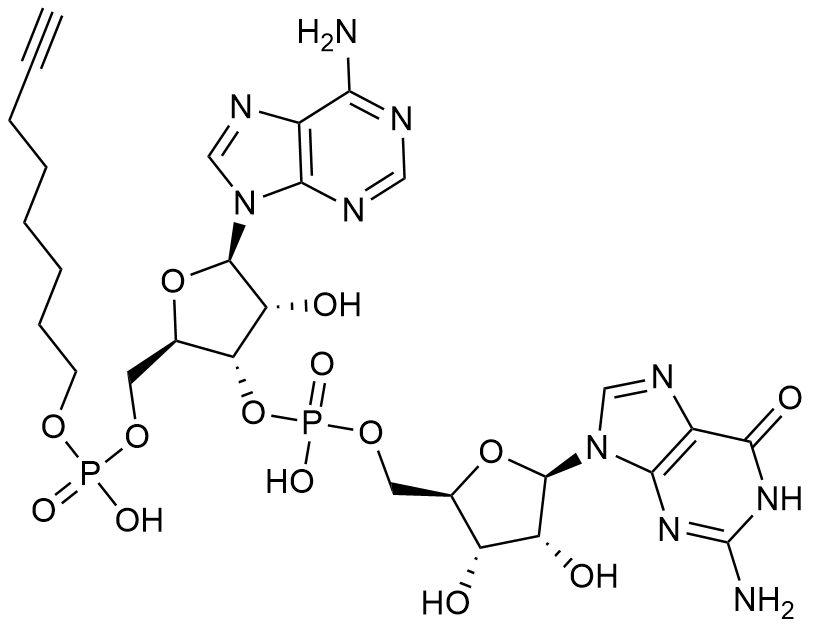
Alkyne-C8-pApG
Innovative 5′ Cap Analog for Enhanced mRNA Translation and Bioorthogonal Conjugation
| Size | Catalog No. | Price |
|---|---|---|
| 1 µmol | BCT-41-S | € 300,00 |
| 5 µmol | BCT-41-L | € 900,00 |
Chemical Properties
-
Molecular Formula
C28H38N10O14P2 (free acid)
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark, dry
-
Molecular Weight
800.61 g/mol (free acid)
-
Purity
≥ 95% (HPLC)
-
Physical State
colorless solution (10 mM)
-
CAS Number
n.a.
-
Absorption (max)
λmax = 256 nm
-
Ɛ (max)
25.050 cm-1M-1
Product Information
Product Overview
Alkyne-C8-pApG is a chemically synthesized cap analog designed for site-specific 5′-end modification of mRNA during in vitro transcription (IVT). This dinucleotide analog introduces a C8-alkyne functional group at the 5′-terminus, enabling precise, bioorthogonal conjugation via click chemistry, including both CuAAC (copper-catalyzed) and SPAAC (copper-free) reactions.
Unlike traditional cap structures that solely enhance translation, Alkyne-C8-pApG serves a dual purpose: it stabilizes mRNA and provides a reactive handle for post-transcriptional modifications. This versatility makes it an invaluable tool for researchers aiming to expand the functional capabilities of synthetic mRNA.
Key Features
- 5′-End Functionalization: Incorporates a C8-alkyne group at the mRNA 5′-end during IVT, facilitating site-specific modifications.
- Bioorthogonal Conjugation: Compatible with click chemistry techniques (CuAAC and SPAAC) for attaching various functional moieties.
- Enhanced mRNA Stability: Contributes to increased mRNA stability, which is crucial for therapeutic applications.
- Versatile Applications: Suitable for mRNA labeling, imaging, and the development of RNA-based therapeutics and gene replacement therapies.
Applications
- mRNA Labeling and Imaging: Enables the attachment of fluorescent dyes or other probes for tracking mRNA within biological systems.
- RNA Therapeutics: Facilitates the conjugation of cell-targeting ligands at the 5′-end, expanding the potential for targeted gene therapies.
- Dual-End Modification: Complements existing 3′-end modification techniques, allowing for comprehensive mRNA functionalization.
- Study of mRNA Dynamics: Assists in investigating mRNA stability, translation efficiency, and interactions with proteins.
Advantages Over Existing Technologies
Traditional mRNA modification strategies predominantly focus on the 3′-end, limiting the scope of functionalization and potentially affecting mRNA stability and translation. Alkyne-C8-pApG addresses these limitations by:
- Enabling 5′-End Modifications: Provides a novel approach to mRNA functionalization, allowing for the attachment of various moieties at the 5′-end without compromising mRNA integrity.
- Facilitating Efficient Conjugation: The alkyne group allows for rapid and specific reactions with azide-containing compounds, streamlining the modification process.
- Enhancing Therapeutic Potential: By enabling the addition of targeting ligands or protective groups at the 5′-end, it broadens the applicability of mRNA in therapeutic contexts.
Summary
Alkyne-C8-pApG represents a significant advancement in mRNA technology, offering researchers a robust tool for 5′-end mRNA modification. Its integration of stability enhancement and functionalization capabilities positions it as a critical component in the development of next-generation RNA-based applications.
For more detailed information, please refer to the data sheet.
LITERATURE
A novel route for preparing 5′ cap mimics and capped RNAs: phosphate-modified cap analogues obtained via click chemistry, S. Walczak et al., 2017, Chemical Science, Vol. 8, p. 260-267

