
Pseudouridine Triphosphate (Pseudo-UTP)
Stabilizing Triphosphate for RNA
| Size | Catalog No. | Price |
|---|---|---|
| 1 µmol | BCT-23-S | € 65,00 |
| 5 µmol | BCT-23-L | € 260,00 |
Chemical Properties
-
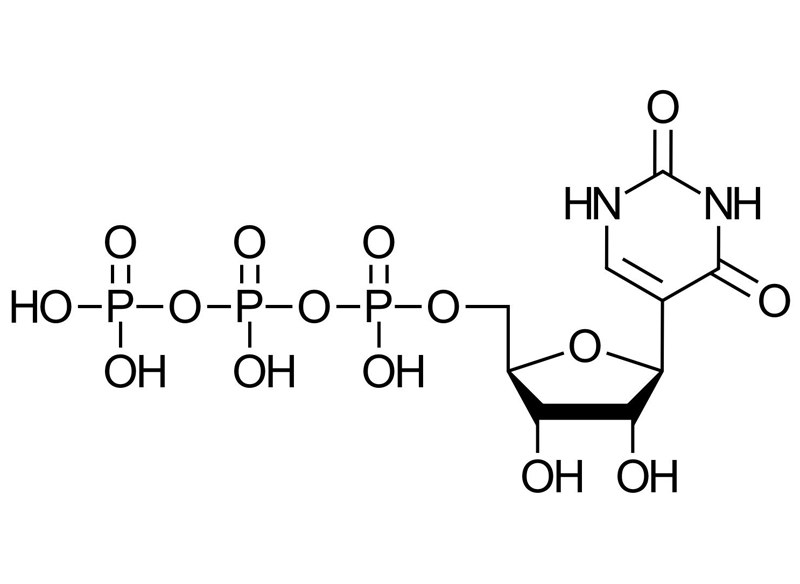
Molecular Formula
C9H15N2O15P3
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C
-
Molecular Weight
484.14 g/mol
-
Purity
≥ 99% (HPLC)
-
Physical State
100 mM clear aquaeous solution; colorless
-
CAS Number
28022-82-4 (sodium salt)
1175-34-4 (free acid)
-
Additional name
5-Ribosyl Uracil, Pseudouridine-5′-triphosphate
-
Absorption (max)
λmax = 262 nm
-
Ɛ (max)
7,500 cm-1M-1
Product Information
A Key Building Block for Immunosilent mRNA
Pseudouridine 5´-triphosphate (ΨTP) is a chemically modified nucleotide often used in synthetic mRNA. This RNA building block is employed in the production of immunosilent mRNA. During in vitro transcription reaction, ΨTP can replace uridine triphosphate (UTP), generating pseudouridine-modified mRNA that evades detection by the innate immune system. This modification enhances mRNA stability and translation efficiency, resulting in a longer half-life time and improved protein expression compared to unmodified mRNA. [1, 2]
Chemical Specificity of the ΨTP:
Normal uridine (U) has a glycosidic bond between the ribose and the nitrogen at position N1 of the uracil base.
Pseudouridine (Ψ) is an isomer where the ribose attaches to C5 of the pyrimidine ring of uracil instead of N1.
This change:
-
- Introduces an extra N-H donor in the base.
- Improves hydrogen bonding and stacking interactions.
- Alters RNA secondary structure flexibility.
Why It Silences Immune Response
- Innate immune sensors (RIG-I, MDA5, TLR7/8) detect foreign RNA patterns, especially:
- Unmodified uridine-rich sequences.
- Structured RNA with exposed 5′ triphosphates.
- Pseudouridine:
- Reduces recognition by TLR7/8 (which bind uridine-rich motifs).
- Alters RNA folding so it looks more like endogenous RNA.
- Minimizes activation of PKR and interferon pathways.
- Result: Lower IFN response, less ISG upregulation, and better translation.
Why buy Pseudouridine 5´-triphosphate from baseclick?
- ≥ 95% HPLC purity for consistent performance
- Market-leading pricing, bulk options, and reliable supply for research applications
- Available sizes: 1 µmol, 5 µmol, and bulk quantities upon request
Applications of Pseudouridine 5′-Triphosphate (ΨTP)
- mRNA Therapeutics and Vaccines: Used in IVT to produce immunosilent mRNA for research on protein expression, gene therapy, and vaccine development.
- Functional Genomics: Enables synthesis of modified mRNA for studying translation efficiency and RNA stability.
- Cell and Molecular Biology: Supports experiments requiring reduced innate immune activation in mammalian systems.
- In Vivo Protein Expression Studies: Ideal for generating mRNA with enhanced half-life for animal models.
Advantages of Using Pseudouridine in mRNA
- Immunosilence: Reduces activation of innate immune sensors (e.g., TLRs), minimizing cytotoxicity and inflammatory responses.
- Improved Stability: Extends mRNA half-life compared to unmodified transcripts.
- Enhanced Translation Efficiency: Promotes higher protein yield in vitro and in vivo.
- Complete Compatibility with IVT: Easily substitutes UTP in standard T7 RNA polymerase-driven transcription.
LITERATURE
[1] Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability, K. Karikó et al., 2008, Molecular Therapy, Vol. 16(11), p. 1833-1840.
https://doi.org/10.1038/mt.2008.200
[2] Pseudouridine RNA avoids immune detection through impaired endolysosomal processing and TLR engagement. M. Bérouti, M. Wagner, W. Greulich, I. Piseddu, J. Gärtig, L. Hansbauer C. Müller-Hermes, M. Heiss, A. Pichler, A. J. Tölke, G. Witte, K.-P. Hopfner, D. Anz, M. Sattler, T. Carell, V. Hornung, 2025, Cell, Vol. 188(18), p. 4880–4895.
[3] Pseudouridine: Still mysterious, but never a fake (uridine). F. Spenkuch, Y. Motorin, M. Helm, 2014, RNA biol., Vol. 11(12), p. 1540–1554.
Activation of Autoreactive B Cells by Endogenous TLR7 and TLR3 RNA Ligands, N. Green et al., 2012, J. Biol. Chem., Vol. 287(47), p. 39789-39799.
https://doi.org/10.1074/jbc.M112.383000
Transient Focal Membrane Deformation Induced by Arginine-rich Peptides Leads to Their Direct Penetration into Cells, H. Hirose et al., 2012, Mol Ther., Vol. 20(5), p. 984-993.
https://doi.org/10.1038/mt.2011.313
Feeder-Free Derivation of Human Induced Pluripotent Stem Cells with Messenger RNA, L. Warren et al., 2012, Sci Rep., Vol. 2, p. 657.
https://doi.org/10.1038/srep00657
Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L, B. Anderson et al., 2011, Nucleic Acids Research, Vol. 39(21), p. 9329-9338.
https://doi.org/10.1093/nar/gkr586
Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA, K. Karikó et al., 2011, Nucleic Acids Research, Vol. 39(21), p. e142.
https://doi.org/10.1093/nar/gkr695
Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation, B. Anderson et al., 2010, Nucleic Acids Research, Vol. 38(17), p. 5884-5892.
https://doi.org/10.1093/nar/gkq347
Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA, L. Warren et al., 2010, Cell Stem Cell, Vol. 7(5), p. 618-630.
https://doi.org/10.1016/j.stem.2010.08.012
Norovirus Proteinase-Polymerase and Polymerase Are Both Active Forms of RNA-Dependent RNA Polymerase, G. Belliot et al., 2005, Journal of Virology, Vol. 79(4), p. 2393-2403.
https://doi.org/10.1128/jvi.79.4.2393-2403.2005
Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA, K. Karikó et al., 2005, Immunity, Vol. 23(2), p. 165-175.
https://doi.org/10.1016/j.immuni.2005.06.008
Pseudouridine in RNA: What, Where, How, and Why, M. Charette et al., 2000, IUBMB Life, Vol. 49(5), p. 341-351.
http://bpg.utoledo.edu/~afedorov/ABPG2011/L23/pseudouridine2000.pdf
Comparative Utilization of Pseudouridine Triphosphate and Uridine Triphosphate by Ribonucleic Acid Polymerase, I. Goldberg et al., 1963, J. Biol. Chem., Vol. 238(5), p. 1793-1800.
https://doi.org/10.1016/S0021-9258(18)81139-5
5-Ethynyluridine: A Bio-orthogonal Uridine Variant for mRNA-Based Therapies and Vaccines, S. Maassen et al., 2023, ChemBioChem, Vol. 24(5), e202200658.
https://doi.org/10.1002/cbic.202200658
Incorporation of Synthetic mRNA in Injectable Chitosan-Alginate Hybrid Hydrogels for Local and Sustained Expression of Exogenous Proteins in Cells, H. Steinle et al., 2018, Int. J. Mol. Sci., Vol. 19(5), p. 1313.
https://doi.org/10.3390/ijms19051313
Prediction of lipid nanoparticles for mRNA vaccines by the machine learning algorithm, W. Wang et al., 2022, Acta Pharmaceutica Sinica B, Vol. 12(6), p. 2950-2962.
https://doi.org/10.1016/j.apsb.2021.11.021
FAQ
-
Is there an IVT kit from baseclick containing pseudouridine 5´-triphosphate?
Yes. Our baseScribe Mod-Pseudo Cap1 Kit enables efficient production of up to 160 µg of Cap1-capped, pseudouridine-modified mRNA per 20 µL reaction from DNA templates containing a T7 RNA polymerase promoter sequence. The resulting immunosilent mRNA, with 100% uridine replacement by pseudouridine, is ready-to-use for protein expression in vitro or in vivo.
-
Does baseclick offer ready-to-use pseudouridine-modified mRNA?
Yes. With our mRNA Service in cooperation with Eurofins Genomics we can provide you with high-quality, ready-to-use mRNA designed exactly for your needs, from native mRNA to highly functionalised mRNA. Our joint mRNA Service includes:
- Plasmid DNA production (via Eurofins)
- High-yield IVT mRNA synthesis (via baseclick)
- 5′-Capping & poly(A) tailing
- Internal base modifications (e.g., pseudouridine, 5-ethynyl uridine)
- Site-specific click conjugation (e.g. with fluorophores, biotin, or other ligands)
- Quality control and data documentation
- Support for downstream LNP encapsulation or in vivo delivery
-
What is the biological function of pseudouridine?
Pseudouridine (Ψ) is a naturally occurring, non-canonical nucleoside which is present in mRNA, tRNA, and other RNA species in all three domains of life. It plays a crucial role in RNA processing and translation life. Pseudouridine is an RNA modification that is done post-transcriptionally, so after the RNA is formed. The enzymes needed to introduce this modification are called pseudouridine synthases (PUS) and are found in all kingdoms of life, indicating that Ψ already was involved in early stages of evolution.

