
X-ClickSeq Library Prep Kit
ClickSeq kit compatible with user-provided primers in reverse-transcription reaction

| Size | Catalog No. | Price |
|---|---|---|
| 12 rxn | BCK-X-RNASeq | € 396,00 |
Chemical Properties
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
– 20 °C
-
Physical State
kit system made of different components
-
CAS Number
n.a.
-
Preparation/Handling
please see user manual of the kit
Product Information
Short Summary:
ClickSeq: A new technique for the preparation of NGS Sequencing Libraries from RNA
ClickSeq is a simple method for the synthesis of Next-Generation Sequencing (NGS) libraries and offers a Next Generation Sequencing (NGS) Library Prep Kit. X-ClickSeq derives its names by using ‘Click-Chemistry‘ in the place of common ligation enzymes to ‘click-ligate’ nucleic acids to sequencing adaptors – an essential and often problematic step of Next Generation Sequencing (NGS) Library Prep Kits and in the synthesis of NGS cDNA libraries. The process takes advantage of the chain-terminating properties of 3′-azido-3`-dioxynucleotides, which are included next to the unmodified NTPs in the initial in vitro reverse-transcription reaction mix uniformly, as required for x-RNAseq technique. The modified nucleotides are stochastically incorporated into the nascent cDNA, yielding cDNA fragments blocked at their 3′ ends with azido groups instead of the natural hydroxyl groups needed for strand elongation. This blockage is similar to the one utilized for Sanger sequencing with the incorporation of 2`-3`-dideoxynucleotides. The 3′-azido-blocked cDNA fragments are ‘click-ligated’ onto alkyne-functionalized sequencing adaptors, which can subsequently be PCR-amplified to yield a sequencing-ready NGS library.
X-ClickSeq kits provide the same reagents as the ClickSeq kits, except that no primers are provided for the Reverse Transcription step. Rather, the user may use their own RT primer designed to the requirements of their own assay. For example, a 9N random primer might be used instead of a 6N random primer included in the ClickSeq kit; or a set of SARS-CoV-2 targeting tiled-primers might be used[1]. All other aspects of the library prep are otherwise identical to ClickSeq. The data analysis pipeline will also be altered as per the assay design.
Problem:
Traditional NGS techniques rely on enzymatic primer ligation for sequencing adaptors, a step that is very prone to errors but needs to be performed with high accuracy to guarantee a reliable NGS library.
Even if the step is performed with high accuracy, there still is the possibility for some DNA fragments to ligate differently resulting in an uneven representation in the library.
The ClickSeq protocol replaces this step by utilizing highly efficient and high yielding Cu(I) catalyzed alkyne azide cycloaddition (CuAAC) for ligation. Therefore, the incorporated 3`-Azido stop nucleotides are click ligated by CuAAC with an alkyne containing adaptor Oligo. This results in a ssDNA molecule with a triazole linked DNA backbone, which is biocompatible for PCR amplification. For the defined 3`end by the stop azido nucleotide, no dual primers for both directions are needed.
These facts in combination with the usage of click chemistry instead of enzymatic ligation also significantly reduce the costs for preparing your NGS library. Additionally, ClickSeq with 6-8 h worktime is a lot faster than comparable procedures.
Workflow of this Next Generation Sequencing (NGS) Library Prep Kit:
-
- Reverse Transcription: Incorporation of 3′-azido nucleotides into the cDNA.
- Click-Ligation: Connection of cDNA fragments with alkyne-functionalized sequencing adaptors.
- PCR Amplification: Amplification of cDNA-adaptor fragments to create the NGS library.
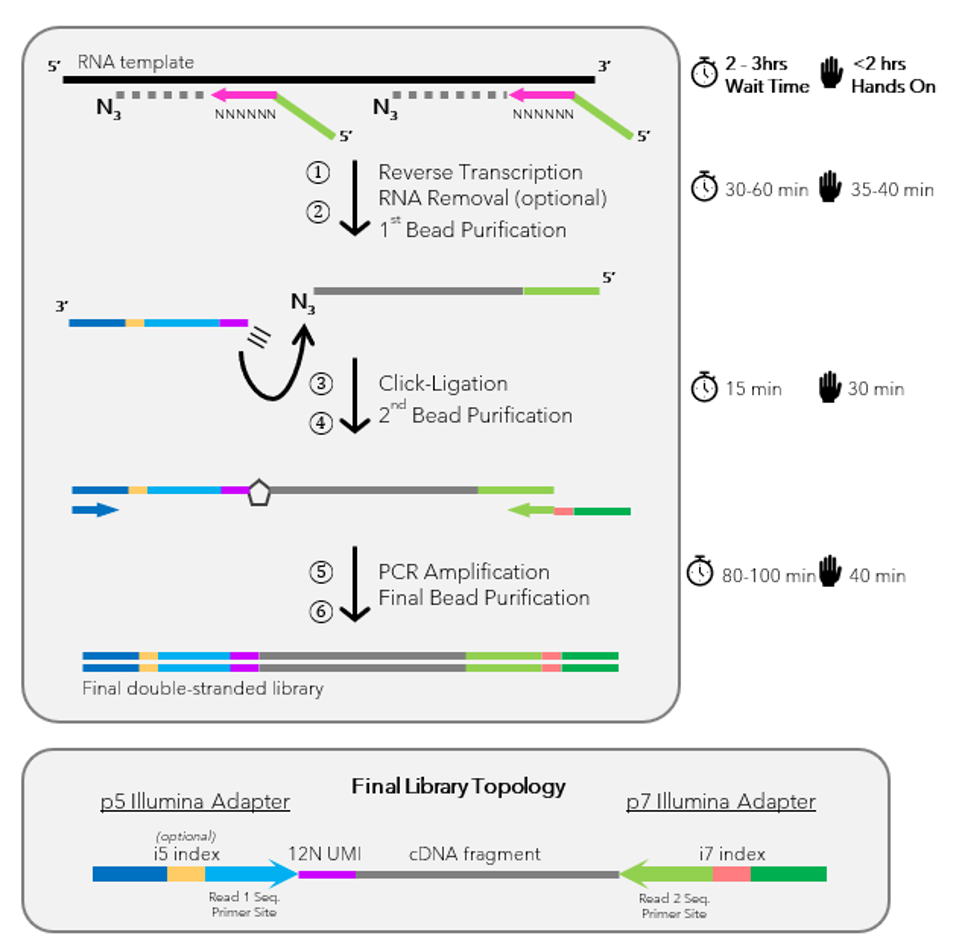
Please note, the x-ClickSeq Library Prep Kit does not contain any Primers for PCR Amplification.
A set of 12 compatible i7 index primers is available at baseclick, which is needed for performing x-ClickSeq sequencing if no user provided Primers should be used instead.
Outcome:
x-ClickSeq is a new technique to produce unbiased NGS libraries with low error rates compared to standard NGS methods. Additionally, Click Seq, utilizing Click Chemistry instead of enzymatic ligation, is robust against common artifacts of NGS such as chimera formation and artifactual recombination with fewer than 3 aberrant events detected per million reads[2].
ClickSeq technology is also available for DNA sequencing and for sequencing of 3`polyadenylated RNA. These kits include primers for in vitro transcriptions.
[1] Jaworski et al, Tiled-ClickSeq for targeted sequencing of complete coronavirus genomes with simultaneous capture of RNA recombination and minority variants. eLife 2021 Sep 28:10:e68479. doi: 10.7554/eLife.68479. PMID: 34581669; PMCID: PMC8478411.
[2] Routh, A., Head, S. R., Ordoukhanian, P., & Johnson, J. E. (2015). ClickSeq: fragmentation-free next-generation sequencing via click ligation of adaptors to stochastically terminated 3′-azido cDNAs. Journal of molecular biology, 427(16), 2610-2616.
https://doi.org/10.1016/j.jmb.2015.06.011
Additional Literature:
Jaworski, E., & Routh, A. (2017). ClickSeq: replacing fragmentation and enzymatic ligation with click-chemistry to prevent sequence chimeras. In Next Generation Sequencing: Methods and Protocols (pp. 71-85). New York, NY: Springer New York.
https://doi.org/10.1007/978-1-4939-7514-3_6
FAQ
-
What is ClickSeq?
ClickSeq is a platform and method for making Next-Generation Sequencing libraries. It is called ‘ClickSeq’ as it replaces the ligations and fragmentation steps common to NGS library preps with ‘Click-Chemistry’.
-
What is X-ClickSeq?
X-ClickSeq kits provide the same reagents as the ClickSeq kit (BCK-RNAseq) but purposefully omit the primers required for the reverse transcription reaction. Rather, the user may use their own RT primer as per the requirements of their own assay. For example, a 9N random primer might be used instead of a 6N random primer; or a set of SARS-CoV-2 targeting tiled-primers might be used. All other aspects of the library prep are otherwise identical to ClickSeq. The data analysis pipeline will also be altered as per the assay design.
-
How does ClickSeq work?
ClickSeq follows the same principle of all NGS library preps that seek to generate short fragments of cDNAs that are flanked with the appropriate sequencing adaptor for the user’s choice of sequencing platform (e.g. Illumina).
The process works by supplementing the Reverse Transcription step of an NGS library prep with small amounts of terminating ‘azido-nucleotides’. These are stochastically incorporated into the cDNA during first stranded synthesis, yielding 3’azido-terminated cDNA molecules. The the azido group is then ‘Click-Ligated’ to an alkyne-functionalized sequencing adaptor using Click-Chemistry (Copper Catalysed Azide-Alkyne Cycloaddition, CuAAC). The resultant click-linked single-stranded cDNA is then PCR amplified to generate a final sequencing-ready NGS library.
-
Can ClickSeq sequence DNA?
Yes. ClickSeq can sequence DNA with the exact same protocol as used for RNAseq provided that the RT enzyme has DNA-templated DNA polymerase activity (such as SuperScript III). Published examples of this can be found here:
https://academic.oup.com/gigascience/article/doi/10.1093/gigascience/giad009/7080818
-
Is ClickSeq published?
Yes. ClickSeq was originally published in 2015 by Routh et al in the Journal of Molecular Biology:
https://www.sciencedirect.com/science/article/abs/pii/S0022283615003514?via%3Dihub
ClickSeq has since been used in numerous publications, by the original inventors, by clients who have purchased ClickSeq kits and/or services as well as independent groups who have assembled their own reagents. See here for a curated list of publications:
-
Where can I find a protocol for ClickSeq?
Our protocol can be found here:
https://www.protocols.io/view/clickseq-random-primed-protocol-with-single-indexi-n92ld8jkov5b/v1
-
What do I need in addition to the kit to make a ClickSeq library?
Required third-party reagents include:
- SuperScript III™ Reverse Transcriptase, 200U/µL (Invitrogen; 18080-093 or 18080-044)
- ClickSeq Library Prep Kit Index Primers (baseclick, BCK-RNAseq-IP)
- [optional] RNaseOUT™ Recombinant Ribonuclease Inhibitor, 40U/µL (Invitrogen; 10777)
- OneTaq® 2X Master Mix with Standard Buffer (NEB; M0482S or M0482L) (Note: you must use OneTaq for this step, this enzyme cannot be substituted for a different PCR enzyme)
- [optional] RNase H, 5000 units/mL (NEB; M0297S or M0297L)
- SPRIselect (Beckman Coulter; B23317) or equivalent DNA/RNA Purification Beads (also known as SPRI beads)
- Nuclease free water
- 80% ethanol (made fresh)
-
Where can I find more information?
Please see an extensive set of FAQs:

