
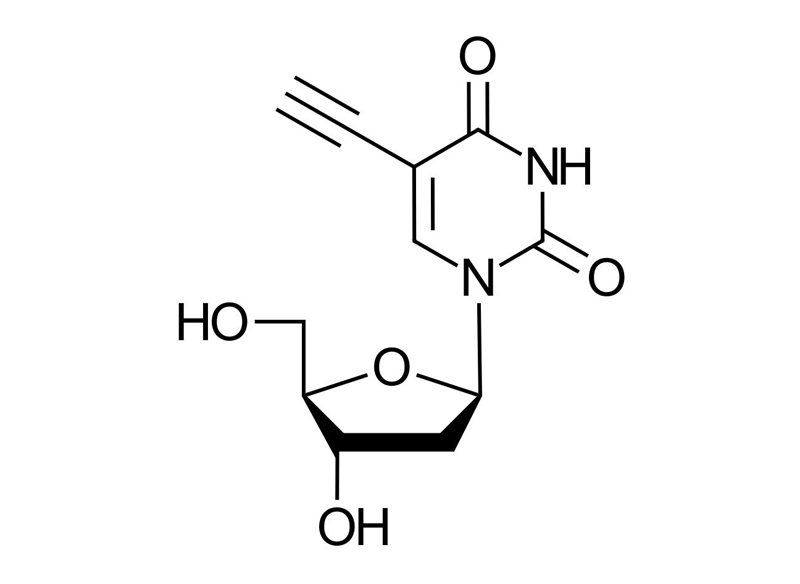
5-Ethynyl-2′-deoxyuridine (5-EdU)
Clickable EdU allows detection of de novo DNA
| Size | Catalog No. | Price |
|---|---|---|
| 50 mg | BCN-001-50 | € 40,00 |
| 100 mg | BCN-001-100 | € 70,00 |
| 500 mg | BCN-001-500 | € 220,00 |
| 1 g | BCN-001-1g | € 375,00 |
Chemical Properties
-
Molecular Formula
C11H12N2O5
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dry
-
Molecular Weight
252.23 g/mol
-
Purity
≥ 98% (HPLC)
-
Physical State
white to off-white solid (water content < 0.5 % w/w)
-
CAS Number
61135-33-9
-
Additional name
EdU, 2′-Deoxy-5-ethynyluridine, 5-Ethynyl-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4(1H,3H)-dione
-
Solubility
DMSO, Alcohol, Water, Aqueous buffers
Product Information
Fast and Sensitive de novo DNA Labeling Without a Denaturation Step
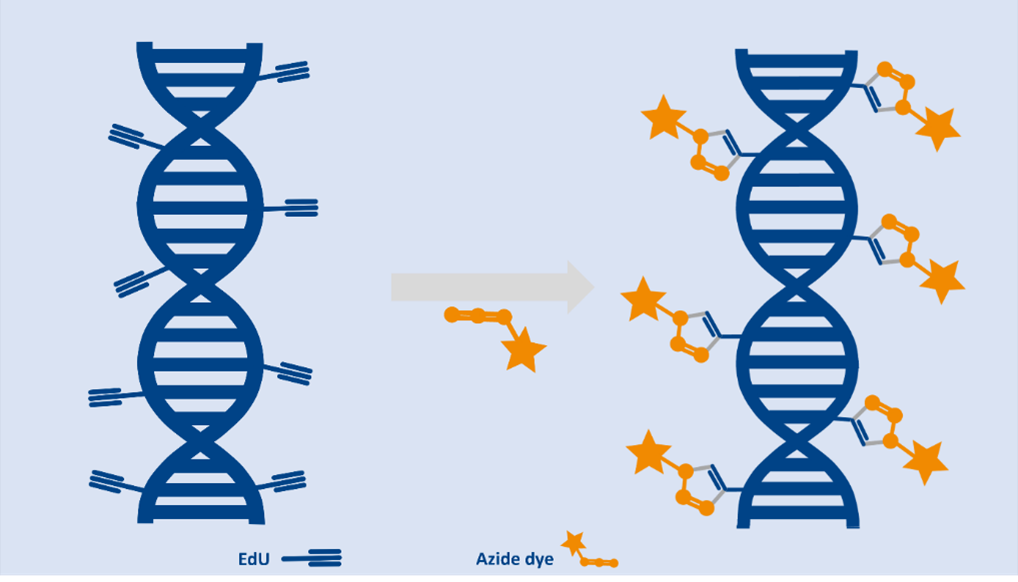
EdU is a thymidine analogue that is incorporated into DNA during S‑phase of the cell cycle. Using copper‑catalyzed azide–alkyne cycloaddition (CuAAC) “click” chemistry, EdU-labeled DNA can be fluorescently tagged without denaturing the DNA, providing clear signals for microscopy, flow cytometry and high-throughput screening, faster and gentler than BrdU.
Why buy 5-EdU from baseclick?
- Market‑leading pricing with bulk options, consistent ≥98% HPLC purity, and rapid, reliable supply for screening and routine proliferation assays.
- Available Sizes: 50 mg, 100 mg, 500 mg, 1 g. Bulk quantities upon request (research use only; not for diagnostic procedures).
What makes 5-EdU different from other options?
- Click‑chemistry detection (CuAAC): Rapid, efficient dye conjugation to the ethynyl handle—recognized in the 2022 Nobel Prize in Chemistry—for high sensitivity and clean backgrounds [1].
- No DNA denaturation: Preserves cell morphology and antigenicity—ideal for multiplexing with immunostaining.
- High signal‑to‑noise ratio: Strong, uniform signals enable robust quantification across fluorescence microscopy, flow cytometry, and HCS/HCA.
- Broad compatibility: Works with mammalian cells, organoids, and tissue sections; supports in vitro and in vivo research workflows [2].
How does 5-ethynyl-2′-deoxyuridine (EdU) work?
Cell‑permeable 5‑EdU is converted intracellularly to the triphosphate and incorporated into newly synthesized DNA, replacing thymidine. The ethynyl group then reacts with azide‑modified fluorophores via CuAAC, generating bright nuclear labeling that marks actively proliferating cells with high specificity and spatial resolution [1, 2].
Fast and ultra-sensitive labeling of proliferating cells with 5-EdU
5-EdU is integral part of baseclick’s Click Tech EdU Cell Proliferation Kits. These kits offer fast, robust and easy-to-use assays for the detection of proliferating cells. They contain all the necessary chemicals for this purpose, including an azido-modified dye of choice. Kits optimised for fluorescence microscopy, flow cytometry and high-throughput screening, both in vitro and in vivo, are available.
Key Features
- Click chemistry-based detection: Enables fast, sensitive, and non-destructive labeling using the copper-catalyzed azide-alkyne cycloaddition (CuAAC).
- No DNA denaturation required: Preserves cell morphology and antigenicity, making it ideal for multiplexing with immunostaining.
- High signal-to-noise ratio: Achieve crisp, reliable results in flow cytometry, microscopy, and high-content screening.
- Broad compatibility: Works seamlessly with mammalian cells, organoids, and tissue sections.
Applications
- Cell proliferation assays (S‑phase labeling)
- Cancer biology & cell cycle studies
- Drug screening & toxicity profiling
- Developmental biology and tissue kinetics
- Stem cell expansion and tracking
LITERATURE
[1] A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes, V. V. Rostovtsev et al., 2002, Angew. Chemie Int. Ed., Vol. 41, p. 2596–2599.
https://doi.org/10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
[2] EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. Chehrehasa et al., J. Neurosci. Methods 2008, 177(1), 122–130.
https://doi.org/10.1016/j.jneumeth.2008.10.006
Template-Directed Oligonucleotide Strand Ligation, Covalent Intramolecular DNA Circularization and Catenation Using Click Chemistry, R. Kumar et al., 2007, J. Am. Chem. Soc., Vol. 129, p. 6859–6864.
https://doi.org/10.1021/ja070273v
Proliferation and Differentiation of Gastric Mucous Neck and Chief Cells During Homeostasis and Injury-induced Metaplasia, J. Burclaff et al., 2020, Gastroenterology, Vol. 158(3), p. 598-609.
https://doi.org/10.1053/j.gastro.2019.09.037
Click Chemistry Generated Model DNA–Peptide Heteroconjugates as Tools for Mass Spectrometry, F. J. Flett et al., 2015, Analytical Chemistry, Vol. 87(19), p. 9595–9599.
https://doi.org/10.1021/acs.analchem.5b02047
FAQ
-
Why is EdU better than BrdU?
EdU detection is antibody‑free and does not require DNA denaturation, preserving epitopes for co‑staining and delivering faster, cleaner readouts.
-
Which readouts are supported?
Fluorescence microscopy, flow cytometry, high‑content imaging; compatible with multiplex immunostaining.
-
Is EdU toxic?
At commonly used concentrations and pulse times, EdU is well‑tolerated in standard cell systems. Titrate for your model and endpoint.

