
DBCO-AF488
Dye to label by SPAAC in copper-free environment
| Size | Catalog No. | Price |
|---|---|---|
| 1 mg | BCFA-237-1 | € 200,00 |
| 5 mg | BCFA-237-5 | € 650,00 |
Chemical Properties
-
Molecular Formula
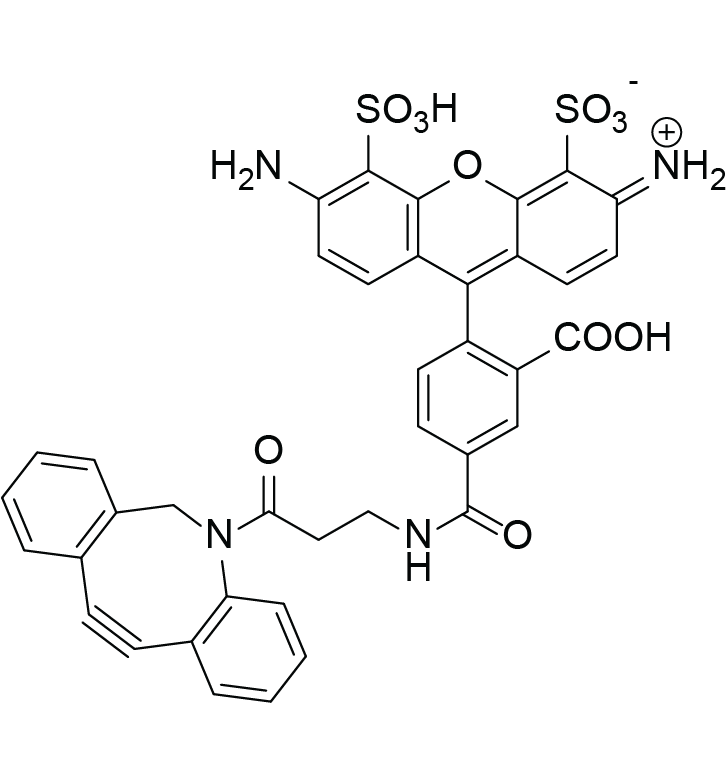
C39H28N4O11S2
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark
-
Molecular Weight
792.8 g/mol
-
Purity
≥ 95% (HPLC)
-
Physical State
orange solid
-
CAS Number
423960-92-1
-
Excitation (max)
495 nm
-
Emission (max)
517 nm
-
Ɛ (max)
73.000 cm-1M-1
-
Solubility
H2O, DMSO, DMF
-
Preparation/Handling
For a 10 mM solution add 126 μL to 1 mg.
For a 10 mM solution add 631 μL to 5 mg.
Product Information
Copper‑Free, 488‑Channel Fluorophore for Bioorthogonal Labeling
DBCO‑AF488 is a green‑fluorescent dye optimized for copper‑free click chemistry. The DBCO (dibenzocyclooctyne) moiety enables strain‑promoted azide–alkyne cycloaddition (SPAAC) with azide‑functionalized targets, eliminating Cu(I) catalysts, ideal for live‑cell labeling and copper‑sensitive systems. Its detection profile matches the 488 nm channel (λex ~495 nm, λem ~517–520 nm), delivering brightness comparable to FAM/FITC‑class dyes and Alexa Fluor 488 workflows.
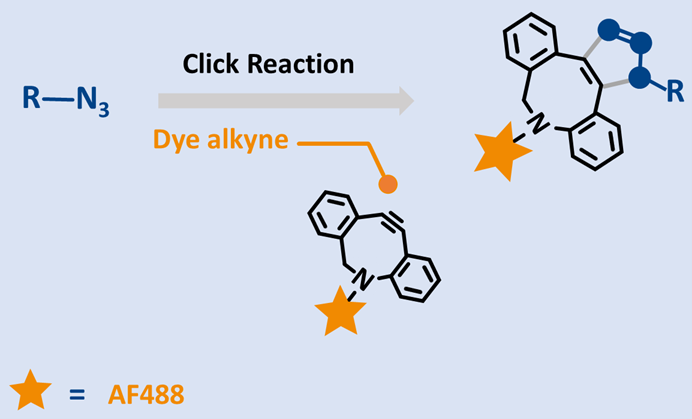
DBCO‑AF488 as a core tool for copper‑free bioorthogonal labeling
DBCO‑AF488 possesses several key features, including:
- High SPAAC reactivity: DBCO provides fast, chemoselective ligation to azides in aqueous buffers under mild conditions.
- Orthogonality in complex systems: Minimal cross‑reactivity with tetrazines enables dual‑label strategies alongside TCO/tetrazine IEDDA chemistry.
- Bright, photostable 488‑nm fluorescence: Sulfonated, hydrophilic dye scaffold supports strong signal and low nonspecific binding.
- Live‑cell and sensitive assay compatible: No copper required; well‑suited for cytotoxicity‑limited or copper‑intolerant workflows.
- Instrument‑friendly: Compatible with standard 488 nm lasers/filters and multiplex panels with FAM/FITC/Alexa Fluor 488.
Applications in Research and Industry
Fluorescence Labeling & Imaging
- Visualization of cellular processes, membrane proteins, and bacterial dynamics with high signal‑to‑background.
Bioorthogonal Conjugation
- Efficient SPAAC labeling of azide‑modified proteins, nucleic acids, glycans, lipids, or nanoparticles with minimal perturbation to native systems.
Advanced Fluorescence Techniques
- High‑content imaging, live‑cell microscopy, and flow cytometry where photostability and copper‑free chemistry are essential.
Multiplex & Workflow Integration
- 488‑channel readout compatible with common multi‑color panels; drop‑in alternative to FITC‑class detection.
Related Products
- DBCO‑AF647: Far‑red (650/668 nm) analog for multiplex imaging with excellent photostability and low background.
- 6‑FAM Azide: Cost‑effective CuAAC label for standard copper‑catalyzed workflows.
6‑FAM‑Picolyl‑Azide and AZDye 488 Azide Plus: Picolyl linker systems that enhance CuAAC kinetics and reduce required copper, benefiting copper‑sensitive samples.
Literature
A metabolic labeling way to in situ fabricate bacterial FRET Platform for innate immune defence molecule, Z. Zhang et al., 2022, Sensors and Actuators B: Chemical, Vol. 350, 130913.
https://doi.org/10.1016/j.snb.2021.130913
Model of collective detachment in high-grade serous ovarian cancer demonstrates that tumor spheroids produce ECM to support metastatic processes, H. M. Micek et al., 2023, APL Bioengineering, Vol. 7(1), 016111.
https://doi.org/10.1063/5.0132254
Investigating Peptidoglycan Recycling Pathways in Tannerella forsythia with N-Acetylmuramic Acid Bioorthogonal Probes, K. A. Wodzanowski et al., 2022, ACS Infect. Dis., Vol. 8(9), p. 1831–1838.

