
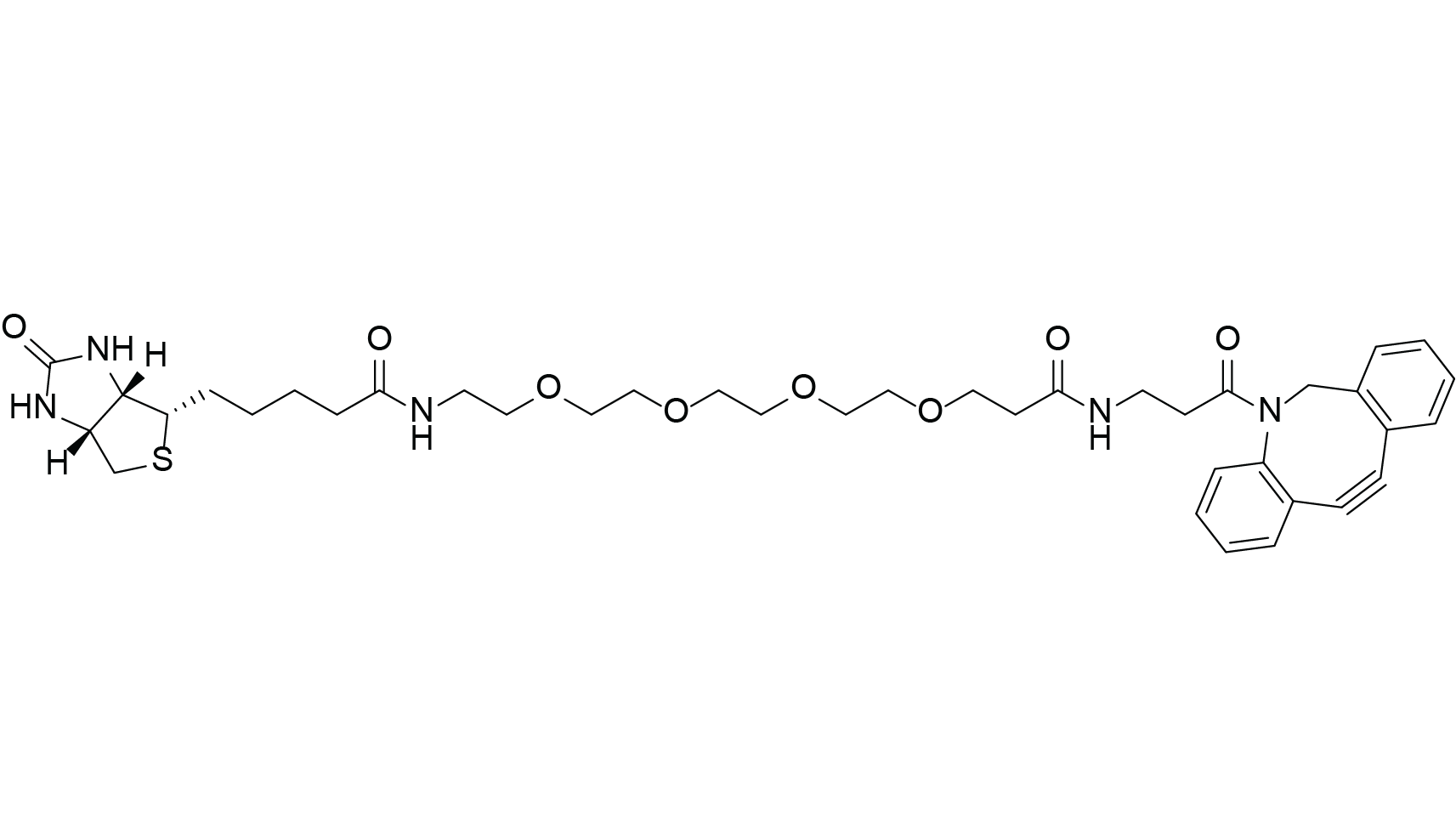
DBCO-PEG4-Biotin
Biotinylation reagent for labeling by SPAAC
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCFA-232-5 | € 110,00 |
| 10 mg | BCFA-232-10 | € 190,00 |
Chemical Properties
-
Molecular Formula
C39H51N5O8S
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark
-
Molecular Weight
749.9 g/mol
-
Purity
≥ 95% (HPLC)
-
Physical State
white to yellow amorphous solid
-
CAS Number
1255942-07-4
-
Solubility
DMSO, DMF, MeOH, DCM, THF
-
Preparation/Handling
For a 10 mM solution add 667 μL to 5 mg.
For a 10 mM solution add 1334 μL to 10 mg.
Product Information
A biotinylation reagent for labeling of azido modified targets by strain-promoted azide alkyne cycloaddition (SPAAC)
What is biotin
Biotin, also known as vitamin B7, is a heterocycle which plays an important role in biological systems. It functions as a coenzyme in carboxylation reactions, for example in fatty acid biosynthesis. In addition to its biological importance, biotin is known for forming one of the strongest non-covalent interactions with the proteins avidin and streptavidin. This exceptionally stable interaction is widely exploited in modern biochemical research, particularly in peptide, protein, and nucleic acid applications.
Applications of biotin
Peptide/protein research:
The biotin–streptavidin (or avidin) interaction enables a wide range of applications in peptide and protein research. Biotinylation is commonly used for affinity purification, where biotin labeled target proteins or protein complexes bind to streptavidin or avidin coated magnetic beads or resins, allowing rapid one step purification.
Biotin labeling is also extensively applied in detection assays through the use of streptavidin conjugated to reporter molecules, most commonly fluorophores or enzymes. This approach is widely used in immuno-assays such as ELISA, Western blotting, and immunohistochemistry. In addition, biotinylation facilitates the enrichment of specific protein populations, for example newly synthesized proteins, for downstream mass spectrometry analysis.
Nucleic acid research:
In nucleic acid research biotin labeling is mostly used for purification processes. Again, the biotin bonding to Streptavidin/Avidin containing beads or resins is utilized to bind the nucleic acids and separate them from impurities. Biotin containing probes can be used for in situ hybridization (ISH) to bind to specific DNA/RNA structures. The probes can be visualized and localized afterwards by binding to Streptavidin/Avidin based markers.
Biotin Labeling via Click Chemistry
All these applications require reliable access to biotin labeled biomolecules. Click chemistry provides a simple, cost effective, and highly selective approach to biotinylation. For click based labeling, the biomolecule of interest must carry either an azide or an alkyne functionality.
Azide or alkyne modified biomolecules, as well as biotinylation reagents such as DBCO-PEG4-Biotin, are commercially available. baseclick offers alkyne or azide modified oligonucleotides and mRNA, as well as pre biotinylated nucleic acids. For other targets or custom applications, feasibility can be discussed via support@baseclick.eu.
Chemical Properties of DBCO-PEG4-Biotin
DBCO-PEG4-Biotin is a highly versatile biotinylation reagent designed for labeling under very mild conditions. Conjugation is achieved via strain promoted azide–alkyne cycloaddition (SPAAC), a copper free click reaction that requires neither heating nor a catalyst, in contrast to CuAAC. The reaction forms a stable triazole linkage with azide modified targets, making it particularly suitable for in vivo labeling applications or other copper sensitive systems.
Azide–alkyne cycloaddition reactions are considered bioorthogonal, as they do not interfere with native biological functionalities. DBCO-PEG4-Biotin further incorporates a hydrophilic PEG4 spacer, which reduces steric hindrance between biotin and the labeled biomolecule while increasing overall water solubility.
These chemical features make DBCO-PEG4-Biotin an ideal choice for biotin labeling of azide modified biomolecules, including peptides, proteins, and nucleic acids, especially when the use of copper must be avoided.
Because click chemistry relies on non-naturally occurring functional groups, the site of biotin attachment can be precisely controlled, in contrast to traditional biotinylation methods such as NHS ester-based chemistry.
LITERATURE
DNA Adduct Detection after Post‐Labeling Technique with PCR Amplification (DNA‐ADAPT–qPCR) Identifies the Pre‐ribosomal RNA Gene as a Direct Target of Platinum–Acridine Anticancer Agents, X. Yao et al., 2021, Chemistry–A European Journal, Vol. 27(59), p. 14681-14689.
https://doi.org/10.1002/chem.202102263
Bisulfite-free, single base-resolution analysis of 5-hydroxymethylcytosine in genomic DNA by chemical-mediated mismatch, Y. Wang et al., 2018, Chemical Science, Vol. 10(2), p. 447-452.
https://doi.org/10.1039/C8SC04272A
O-GlcNAcylation of blimp-1 in lymphocytes inhibits its transcriptional function and is associated with migration and invasion of breast cancer cells, Y. F. Chen et al., 2022, Molecular Cancer Research, Vol. 20(4), p. 650-660.
https://doi.org/10.1158/1541-7786.MCR-21-0405
FAQ
-
What is DBCO‑PEG4‑Biotin used for?
DBCO‑PEG4‑Biotin is used to biotinylate azide‑modified biomolecules via strain‑promoted azide alkyne cycloaddition (SPAAC). This copper‑free click reaction enables highly selective, gentle, and bioorthogonal labeling of peptides, proteins, nucleic acids, and other azide‑functionalized targets.
-
What is the advantage of using DBCO‑PEG4‑Biotin over traditional biotinylation methods?
Unlike NHS‑ester biotinylation, SPAAC labeling with DBCO‑PEG4‑Biotin:
- does not modify lysines or amino groups
- provides site‑specific biotin attachment
- avoids over‑labeling and loss of protein activity
- works under mild, aqueous, copper‑free conditions
This results in cleaner labeling and improved compatibility with sensitive biological samples.
-
Why is DBCO used for labeling?
DBCO is a strained cyclooctyne that reacts rapidly with azides without requiring copper. Benefits include:
- fast reaction kinetics
- no need for catalysts or heat
- suitability for in vivo or copper‑sensitive environments
- stable triazole linkage formation
SPAAC with DBCO is one of the most reliable bioorthogonal reactions for live‑cell and in‑solution labeling.
-
What is the function of the PEG4 spacer?
The PEG4 linker improves performance by:
- reducing steric hindrance between biotin and the target molecule
- increasing solubility in aqueous buffers
- improving accessibility of biotin for streptavidin binding
- minimizing nonspecific interactions
This makes labeling more efficient, especially for large biomolecules.
-
Which biomolecules can be labeled with DBCO‑PEG4‑Biotin?
Any biomolecule that contains an azide group can be labeled. This includes:
- azido‑modified peptides and proteins
- azide‑functional DNA, RNA, oligos, and mRNA
- glycans and polysaccharides
- viruses and virus‑like particles
- nanoparticles or surfaces presenting azides
baseclick also provides pre‑functionalized nucleic acids and custom modifications upon request.
-
Why is biotin important in biochemical workflows?
Biotin forms an exceptionally strong non‑covalent interaction with streptavidin or avidin. This interaction is widely used for:
- affinity purification
- pull‑down assays
- ELISA and Western blot detection
- immunohistochemistry
- protein–protein interaction studies
- nucleic acid capture, ISH, Southern and Northern blots
The strength and specificity of the biotin‑streptavidin complex make it a universal molecular handle.
-
Is DBCO‑PEG4‑Biotin suitable for in vivo labeling?
Yes. SPAAC is copper‑free, making DBCO‑PEG4‑Biotin compatible with:
- live‑cell labeling
- in vivo animal studies
- labeling copper‑sensitive enzymes or proteins
- applications where copper ions would be toxic or disruptive
Its mild reaction conditions preserve native biomolecular function.
-
What reaction conditions are required for SPAAC biotinylation?
SPAAC requires only:
- an azide‑modified target
- DBCO‑PEG4‑Biotin
- aqueous buffer (no copper, no heating, no special catalysts)
The reaction proceeds rapidly at room temperature, forming a stable triazole linkage.
-
How stable is the resulting biotin conjugate?
The triazole linkage formed via SPAAC is exceptionally robust and stable under:
- physiological conditions
- heat
- enzymatic environments
- common biochemical buffers
This makes the labeling suitable for demanding applications such as pull‑downs, imaging, and protein interaction studies.
-
How should DBCO‑PEG4‑Biotin be stored?
Store at –20 °C, protected from moisture and light. Avoid repeated freeze‑thaw cycles to maintain reactivity.
-
Does baseclick offer complementary azide‑modified biomolecules?
Yes. baseclick provides:
- azide‑modified oligonucleotides and mRNA
- pre‑biotinylated nucleic acids
- DBCO‑modified nucleic acids
- custom synthesis of azide or alkyne‑functional targets
For special applications, contact support@baseclick.eu.
-
When should I use DBCO‑PEG4‑Biotin instead of Biotin‑PEG4‑Azide or Biotin‑Azide Plus?
Use DBCO‑PEG4‑Biotin when:
- your target contains an azide
- you require copper‑free biotinylation
- you are working with sensitive proteins, live cells, or in vivo models
- you want fast, clean, bioorthogonal labeling
Use Biotin‑PEG4‑Azide when your target carries an alkyne or strained alkyne (SPAAC or CuAAC).
Use Biotin‑Azide Plus when you perform CuAAC with minimal copper.

