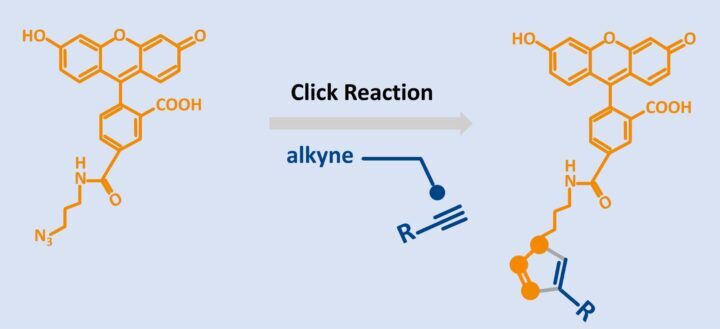
Fluorescein Azide (6-FAM-Azide)
FITC - Fluorescent dye to label DNA/RNA
| Size | Catalog No. | Price |
|---|---|---|
| 5 mg | BCFA-001-5 | € 80,00 |
| 10 mg | BCFA-001-10 | € 115,00 |
Chemical Properties
-
Molecular Formula
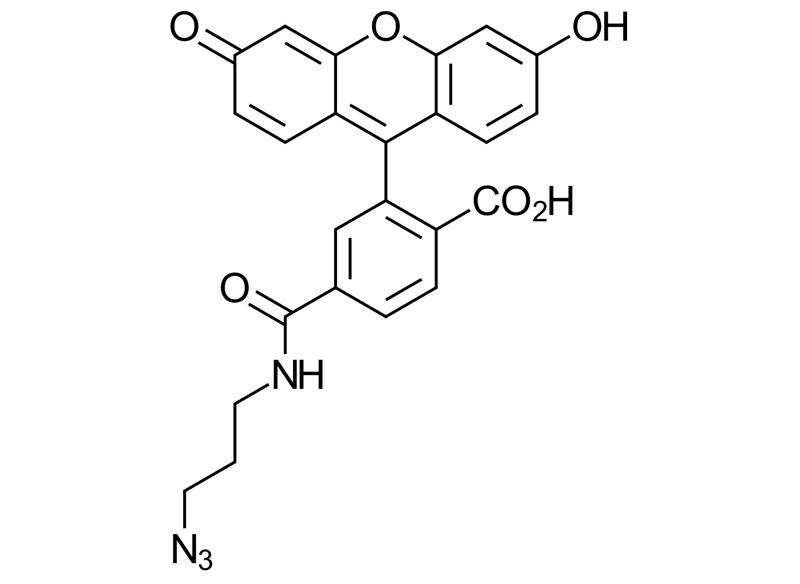
C24H18N4O6
-
Shelf Life
12 months unopened after receipt
-
Storage Conditions
-20 °C, dark
-
Molecular Weight
458.43 g/mol
-
Purity
≥ 95% (HPLC)
-
Physical State
yellow to orange solid
-
CAS Number
1386385-76-7
-
Additional name
6-FAM azide; 6-Carboxyfluoresceine Azide, 4-((3-Azidopropyl)carbamoyl)-2-(6-hydroxy-3-oxo-3H-xanthen-9-yl)benzoic acid; N-(3-azidopropyl)-3′,6′-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9′-xanthene]-6-carboxamide
-
Excitation (max)
50 mM PBS, pH 9: 496 nm
-
Emission (max)
50 mM PBS, pH 9: 516 nm
-
Ɛ (max)
50 mM PBS, pH 9: 83,000 cm-1M-1
-
Solubility
DMSO, DMF, MeOH
-
Preparation/Handling
For a 10 mM solution add 218 μL to 1 mg.
Product Information
Click‑Ready Green Fluorophore for Bioorthogonal Labeling
Fluorescein Azide (6‑FAM‑Azide), also known as 6‑Carboxyfluorescein Azide, is a green, fluorescent dye widely used for biomolecule labeling via click chemistry. With an excitation maximum near 495 nm and emission around 520 nm, it delivers bright, stable fluorescence compatible with FITC, ATTO 488, Alexa Fluor 488, and DyLight 488 detection channels. Its high quantum yield and photostability make it a trusted choice for imaging, diagnostics, and advanced bioconjugation workflows.
Key advantages that make 6‑FAM‑Azide indispensable in biorthogonal chemistry
6‑FAM‑Azide enables clean, efficient conjugation under mild conditions, revolutionizing click‑based labeling strategies.
- High fluorescent quantum yield: Produces strong, reliable green signal for sensitive detection.
- Mild, bio‑orthogonal reactivity: Ideal for CuAAC click chemistry with minimal biological interference.
- Stability under physiological conditions: Maintains fluorescence integrity in live‑cell and in‑vitro systems.
- Exceptional photostability: Resists photobleaching for extended imaging sessions.
- Chemical robustness: Performs consistently across diverse experimental setups.
Applications in Research and Industry
6‑FAM‑Azide is a versatile labeling reagent across multiple scientific fields:
Bioconjugation
- Labels DNA, RNA, proteins, and carbohydrates with bright, photostable fluorescence.
Bio‑orthogonal Labeling
- Azide group enables selective conjugation to alkynes via click chemistry for precise, site‑specific labeling.
Drug Discovery & Cellular Imaging
- Supports live‑cell fluorescence microscopy and high‑content screening with strong signal and low background.
Diagnostics & Biosensing
- Integral to fluorescence‑based assays, biosensor development, and molecular diagnostics.
Forensic & Environmental Analysis
- Applied in DNA fingerprinting and biomarker detection for environmental monitoring.
Agricultural Biotechnology & Nanotechnology
- Useful in crop genetics, biosensing, and nanoparticle functionalization.
Surface Immobilization
- Enables stable fluorescent tagging on alkyne‑functionalized surfaces for sequencing and bioassay platforms.
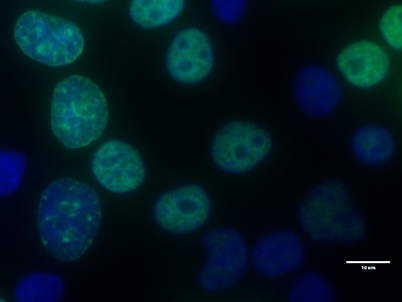
Fluorescence imaging of replicating HeLa cells detected with the EdU Cell Proliferation Kit for Imaging (BCK-EdU488IM100). Green signal: EdU labelling with 6‑Carboxyfluorescein Azide using click reaction; blue signal: DNA counterstaining with DAPI; scale bar = 10 µm. Distinct replication patterns allow staging of replicating cells along S phase (early/mid/late S phase).
Fluorescein Azide (6-FAM-Azide) remains a valuable tool in modern biochemical research and diagnostics, offering versatility and reliability for fluorescence-based methodologies.
In addition to 6-FAM-Azide, baseclick is pleased to offer our customers AZDye 488 Azide plus and 6-FAM-Picolyl-Azide, highly photostable, water-soluble green-fluorescent dyes optimized for click chemistry applications. Their advanced copper-chelating system ensures efficient CuAAC reactions with minimal copper usage, making it ideal for labeling copper-sensitive biomolecules. AZDye 488 Azide Plus and 6-FAM-Picolyl-Azide deliver fluorescence detection comparable to FAM, FITC, and other widely used dyes, ensuring seamless integration into diverse experimental workflows.
CuAAC reaction between AZDye 488 Azide plus and alkyne functionalized molecule
Literature
Postsynthetic DNA Modification through the Copper-Catalyzed Azide–Alkyne Cycloaddition Reaction, P. M. E. Gramlich et al., 2008, Angew. Chemie Int. Ed., Vol. 47, p. 8350–8358.
https://doi.org/10.1002/anie.200802077
Click–Click–Click: Single to Triple Modification of DNA, P. M. E. Gramlich et al., 2008, Angew. Chemie Int. Ed., Vol. 47, p. 3442-3444.
https://doi.org/10.1002/anie.200705664
Synthesis of hemicyanine dyes for ‘click’ bioconjugation, W. H. Zhan et al., 2005, Tetrahedron Lett., Vol. 46, p. 1691–1695.
https://doi.org/10.1016/j.tetlet.2005.01.066
DNA visualization in single molecule studies carried out with optical tweezers: Covalent versus non-covalent attachment of fluorophores, S. Suei et al., 2015, Biochemical and Biophysical Research Communications, Vol. 466(2), p. 226-231.
https://doi.org/10.1016/j.bbrc.2015.09.013
Collinearity of homoeologous group 3 chromosomes in the genus Hordeum and Secale cereale as revealed by 3H-derived FISH analysis, L. Aliyeva-Schnorr et al., 2016, Chromosome Research, Vol. 24, p. 231–242.
https://doi.org/10.1007/s10577-016-9518-8
Cohesin depleted cells rebuild functional nuclear compartments after endomitosis, M. Cremer et al., 2020, Nature Communications, Vol. 11, p. 6146.