2′,3′-dideoxyguanosine triphosphate (ddGTP) is a modified nucleotide analog that acts as a chain-terminating inhibitor in DNA synthesis reactions. This property is used in Sanger sequencing and other biochemical assays.
Structurally, ddGTP resembles the natural deoxynucleotide dGTP but lacks hydroxyl (-OH) groups at both the 2′ and 3′ positions of its deoxyribose sugar ring preventing further chain extension after incorporation by DNA polymerase opposite a template cytosine (C). This creates truncated DNA fragments ending with guanosine (G).
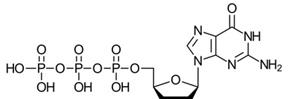
Figure 1. Structural formula of 2′,3′-dideoxyguanosine triphosphate (ddGTP)
Role in Chemo-Enzymatic Labeling
Chemo-enzymatic labeling processes combine enzymatic reactions with chemical modifications (such as azides or alkynes for click chemistry) to attach labels to nucleic acids. The role of modified ddGTP based triphosphates in these reactions is primarily to act as a chain terminator, enabling the controlled, site-specific addition of a single nucleotide to the 3′ end of DNA or RNA strands using enzymes such as terminal deoxynucleotidyl transferase (TdT). This prevents further extension and enables precise labeling.
Learn more about Click Chemistry Glossar here: Click Chemistry Glossar
Chemically modified ddGTP (e.g., with an azide group as in 3′-Azido-2’3′-ddGTP) acts as the labeling substrate itself. TdT incorporates the modified ddGTP at the 3′ end, thereby terminating the chain and introducing a functional handle (azide) for the subsequent chemical conjugation of fluorescent dyes or other tags via click reaction. This is particularly useful for mRNA labeling in studies or next-generation sequencing (NGS) library preparation. baseclick developed a protocol using copper catalysed alkyne azide cycloaddition (CuAAC) for mRNA labeling which shows almost no degradation of the nucelic acid. As alternative we also provide a protocol for strain promoted alykne azide cycloadditon (SPAAC) if a copper free environment is preferred.
Overall, the chain-terminating property of ddGTP and its analogs is essential for achieving single-nucleotide precision in enzymatic labeling. This is complemented by chemical steps that attach diverse labels without compromising the integrity of the nucleic acid.
Benefits
ddGTP has several advantages over conventionally labeled nucleotides:
- Better Enzymatic Incorporation: unlike bulky pre-labeled nucleotides, modified ddGTP has small functional groups that enable it to be efficiently incorporated by enzymes such as TdT.
- Flexible Labeling: the two-step process (enzymatic incorporation followed by chemical labeling via click chemistry) enables a variety of labels to be added after incorporation. This is in contrast to fixed-label nucleotides, which require new synthesis for each label.
- Lower Background Noise: unlike pre-labeled nucleotides, which are prone to background noise, post-incorporation washing and specific click chemistry reduce non-specific signals.
- Precise Labeling: unlike conventional nucleotides that may allow multiple incorporations, ddGTP chain termination ensures single-nucleotide addition at the 3′ end, making it ideal for mRNA or NGS applications.
- Cost-Effective: using one modified ddGTP for multiple labels is cheaper and more scalable than synthesizing various pre-labeled nucleotides. Pre-labeled nucleotides are also incorporated worse due to their bulky labeles.
- Application Compatibility: Precise labeling is suited to sensitive techniques such as single-molecule imaging, whereas bulky conventional labels may interfere with nucleic acid function.
Advantages of using ddGTP in research workflows
Key advantages of ddGTP for research workflows:
- Compatibility: modified ddGTP analogue 3′-azido-ddGTP is effective with enzymes such as TdT and bioorthogonal reactions such as click chemistry, enabling a variety of applications (e.g. NGS and mRNA labeling).
- Flexible Integration: ddGTP can be incorporated into a variety of workflows (e.g. sequencing and imaging) and allows for diverse labeling without the need for protocol changes.
- Modular Design: enzymatic incorporation and chemical labeling enable the attachment of customizable labels using one ddGTP variant for multiple experiments.
- Increased Efficiency: small modifications ensure high incorporation yield, low background noise via specific chemistry as well as cost savings by avoiding the need for multiple nucleotide synthesis.
ddGTP applications in Science & Diagnostics
Common applications of ddGTP include:
- End-Labeling of RNA and ssDNA: adding of modified ddGTP (e.g. 3′-azido-ddGTP) to the 3′ ends via TdT to enable precise tagging with fluorophores for use in imaging or structural studies.
- Preparation for Ligation and Circularization: capping on the 3′ ends block extension, enabling controlled ligation or the formation of circular RNA/DNA for stability or synthetic biology purposes.
- Sequencing Library Prep: modification of the 3′ ends of fragments for the addition of adapters and barcodes in NGS workflows (e.g. Illumina), improving accuracy and yield. baseclick is providing a solution kit for full length mRNA sequencing based on the incorporation of 3`-azido-ddGTP to cDNA.
- Diagnostic Assay Development: It targets labeling for sensitive detection in qPCR and fluorescence assays, as well as pathogen tests, thereby enhancing specificity and reducing false positives.
ddGTP compared to other technologies & methods
ddGTP-based labeling (chemo-enzymatic, e.g., using 3′-azido-ddGTP with TdT) differs from other nucleic acid labeling methods like nick translation, random priming, chemical modification (e.g., NHS ester), and 5′ end-labeling (e.g., T4 PNK):
- Mechanism: ddGTP enables enzymatic chain termination for single-nucleotide addition at 3′ ends, followed by click chemistry for labeling. Nick translation and random priming incorporate multiple labeled dNTPs during DNA synthesis. Chemical modification covalently attaches tags (e.g. via amine groups) and 5′ end labelling uses T4 polynucleotide kinase to add a phosphate group or a label to the 5′ end.
- Precision and Specificity: ddGTP provides 3′ end-specific, single-nucleotide labeling, that is ideal for mRNA or NGS. In contrast, nick translation labels every 20–25 nucleotides, random priming adds labels variably along strands, chemical modification lacks sequence specificity and 5′ end labelling only targets 5′ ends.
- Efficiency and Compatibility: The small azide of azide-modified ddGTP ensures high compatibility with TdT polymerase. In nick translation or random priming, bulky labeled dNTPs reduce incorporation efficiency. Chemical modification requires purified nucleic acids, while 5′ end-labeling is enzyme-specific but limited to 5′ ends.
- Flexibility: The ddGTP-based two-step process enables the attachment of various labels after incorporation. Nick translation and random priming require pre-labelled dNTPs per tag. Chemical modification is less adaptable, with 5′ end labeling restricted to specific 5′ modifications.
In short, ddGTP provides precise, efficient 3′ end-labeling for diagnostics or sequencing, outperforming nick translation, random priming, chemical modification, and 5′ end-labeling in specificity and flexibility.
The niche role of ddGTP in molecular biology workflows is defined by its unique chemical structure and its ability to terminate DNA synthesis.
Niche Role of ddGTP in Molecular Biology:
- Chain Termination in DNA Sequencing that allows precise mapping of DNA sequences by generating fragments of varying lengths.
- Chemo-enzymatic labeling uses modified ddGTPs (e.g., 3′-Azido-2′,3′-ddGTP) which are incorporated into DNA by polymerases. The azido group enables click chemistry, allowing the attachment of fluorescent dyes, biotin and affinity tags. This process is employed in DNA visualisation, pull-down assays and single-molecule studies.
- Selective DNA termination for probe design: ddGTP can be used to design probes that hybridize to specific sequences and terminate extension. This is useful detectinig mutation, genotyping, and allele-specific PCR.
- Studying polymerase fidelity: the incorporation of ddGTP helps to assess the accuracy with which DNA polymerases replicate DNA. It is used for enzyme engineering and drug development.
- Epigenetic and transcriptomic applications: In workflows where DNA synthesis is being tracked (e.g. nascent strand labelling), ddGTP variants can be used to mark the newly synthesised DNA.
The science behind ddGTP & click chemistry
Click chemistry refers to a class of modular, efficient reactions that join molecules under mild conditions with high yield, specificity, and minimal byproducts. Introduced by K. Barry Sharpless, it emphasises bioorthogonal reactions, which are compatible with biological systems without causing interference, for applications such as bioconjugation and labeling. This concept won the Nobel Prize in Chemistry in 2022.
Copper-catalyzed azide-alkyne cycloaddition (CuAAC) involves a [3+2] cycloaddition reaction between an azide (R-N₃) and a terminal alkyne (R’-C≡CH), catalyzed by Cu(I), forming a stable 1,4-disubstituted 1,2,3-triazole ring. A variant is strain-promoted azide-alkyne cycloaddition (SPAAC), which can be used in copper-free applications.
Azide-alkyne cycloaddition enables the efficient labeling of nucleotides by allowing the bioorthogonal conjugation of functional groups (e.g., fluorophores or biotin) to nucleotides without disrupting their biological activity. Small azide or alkyne handles are incorporated into nucleotides and then ‘clicked’ with complementary tags to ensure high specificity and yield (>90%) with minimal steric hindrance.
3′-Azido-2′,3′- ddGTP is incorporated into the 3′ end of DNA or RNA by terminal deoxynucleotidyl transferase (TdT), which terminates chain extension. CuAAC then conjugates the azide with an alkyne-bearing label (e.g., a fluorescent dye), enabling precise, site-specific labeling for applications such as sequencing, imaging, or diagnostics.
Practical guide: How to use ddGTP effectively
Practical Guide for ddGTP in Chemo-Enzymatic Labeling:
- Enzyme Selection: use DNA polymerases that tolerate modified nucleotides. Terminal deoxynucleotidyl transferase (TdT), for example, is the standard choice for incorporating modified ddGTP (e.g. 3′-azido-2′,3′- ddGTP) at the 3′ end of DNA or RNA, as it is template-independent and efficiently adds single ddNTPs to prevent extension. Commercial TdT is available. As an alternative, reverse transcriptase (RT) or the Klenow fragment (exo–) can be used for cDNA labeling, but TdT is preferred for precise 3′ tailing. Avoid enzymes with strict substrate specificity.
- Reaction Conditions for ddGTP Incorporation
- Buffer: Use enzyme-specific buffer with Mg²⁺ or Mn²⁺ which is usually provided with the enzyme.
- Temperature: Typically 37°C for TdT or Klenow.
- Time: 30–60 minutes depending on enzyme and DNA length.
- ddGTP concentration: 10–1000 µM is common.
- Combining with Alkyne-Modified Labels (CuAAC)
After ddGTP incorporation (with azide group), perform CuAAC click reaction:
- Add alkyne-modified dye or biotin.
- Use CuSO₄ + sodium ascorbate or a commercial Cu(I) catalyst.
- Include ligands like THPTA to stabilize Cu(I) and prevent side reactions.
- Incubate at room temp or at 37°C for 30–90 min (or overnight 12-17 h for higher yield)
Alternative, use copper-free variants (e.g., SPAAC with cyclooctyne) if Cu toxicity is a concern.
- Troubleshooting Tips:
- Low labeling efficiency: Check enzyme activity, ddGTP purity, and DNA quality.
- Non-specific labeling: Optimize CuAAC conditions and wash steps.
- Poor incorporation: Try different polymerases or adjust metal ion concentration.
- DNA degradation: Minimize Cu exposure time and use stabilizing ligands.
baseclick GmbH offers a wide range of innovative products and services centered around click chemistry for DNA, RNA, and mRNA applications:
- ClickSeq Library Prep Kits is a novel approach to Next-Generation Sequencing (NGS) library preparation that replaces enzymatic ligation with a bioorthogonal chemical reaction, specifically, copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC).
- Click-compatible nucleotides: Beyond 3′-Azido-2′,3′-ddGTP, baseclick provides a range of azide- and alkyne-modified nucleotides for DNA, RNA, and mRNA labeling.
- Modified mRNA synthesis: ddGTP is used for 3′-end labeling of mRNA facilitating site-specific tagging without altering transcription protocols.
- Ligand-mediated RNA targeting: ddGTP-labeled RNA can be conjugated to ligands (e.g. sugars, amino acids) via click chemistry supporting targeted delivery and therapeutic applications.
Learn more about RNA Delivery Glossar here: RNA Delivery Glossar

